
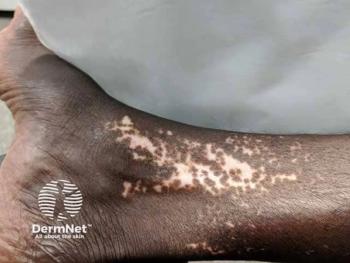
Discover how anatomical variations impact the minimal erythema dose in 308-nm excimer laser therapy for vitiligo in Asian patients.
Maddi Hebebrand is an associate editor of Dermatology Times and joined the MJH Life Sciences team in May 2024. She attended Baldwin Wallace University, studying Media Production and Film, and received her Masters in Digital Media from Ohio University. When she's not writing, Maddi loves to read, attend concerts and spend time with her family.

Discover how anatomical variations impact the minimal erythema dose in 308-nm excimer laser therapy for vitiligo in Asian patients.
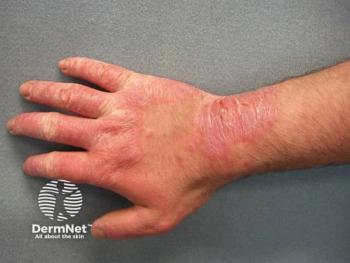
Comprehensive patch testing is vital for accurately diagnosing various forms of dermatitis, improving patient care, and addressing public health challenges.

MediWound reveals clinical data showing NexoBrid significantly reduces pigmentation in traumatic tattoos from abrasions and blast injuries.
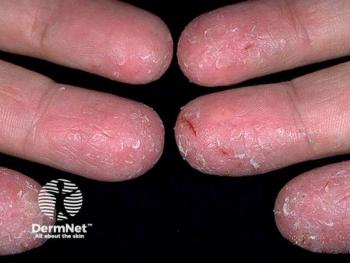
Explore the emotional and physical challenges of chronic hand eczema through a patient's journey and expert insights on empathetic care and innovative treatments.

Discover the efficacy and safety of topical roflumilast 0.3% for psoriasis, highlighting its rapid results and potential for sensitive skin areas.
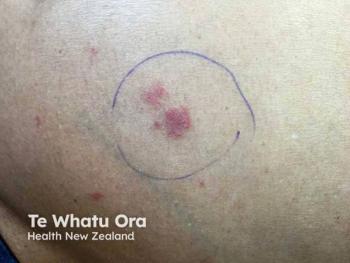
Kymera Therapeutics reveals promising phase 1b results for KT-621, an oral STAT6 degrader, showing significant effects in moderate to severe atopic dermatitis.

Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.

A study reveals high rates of intimate partner violence among women with psoriasis, linking it to poorer quality of life and increased PTSD symptoms.

Experts explore the integration of AI in atopic dermatitis management, highlighting benefits, risks, and the need for cautious implementation in patient care.
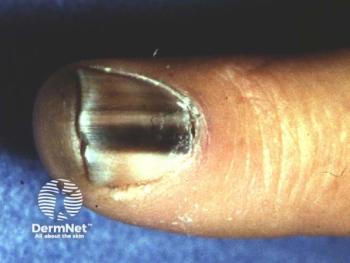
Alpha-9 Oncology's A9-3408 trial targets the melanocortin 1 receptor (MC1R) in advanced melanoma, offering a novel radiotherapeutic approach beyond traditional treatments.
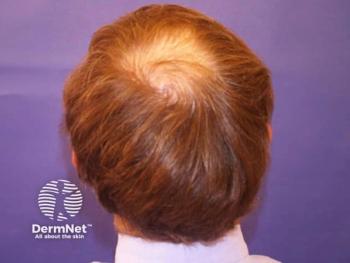
Cosmo Pharmaceuticals reveals promising phase 3 results for clascoterone 5% solution, showing significant hair growth improvement in alopecia.

Tori Spelling and Stella McDermott share their journey with eczema, promoting the Free to Be Me campaign and discussing innovative treatments like roflumilast.
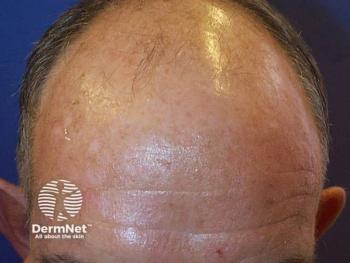
Discover how a proprietary extract from saw palmetto promotes hair growth and reduces thinning in adults, offering a safe alternative to traditional treatments.

The study provides new evidence beyond previously established associations with breast, ovarian, and uterine cancers.

Navigator Medicines advances NAV-240, a promising bispecific antibody for HS, targeting dual pathways for enhanced treatment efficacy.
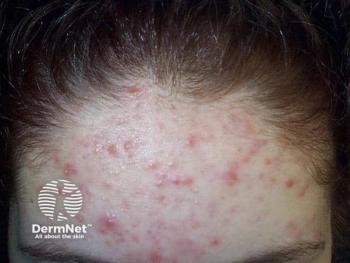
However, over half of the patients reported experiencing psychological symptoms such as mood changes and depression during treatment.

Participants agreed that HS is rarely controlled with monotherapy and often requires coordinated multimodal care.

Support vital dermatology organizations this Giving Tuesday and beyond to enhance patient care, drive research, and foster community.

While individual fatty acids in tallow have known biological effects, their combined impact in whole tallow remains uncertain.

Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.

Explore the top headlines of the month, including insights on regulatory updates, expert pearls, and more.
Hair growth, density, and aging patterns differ across populations, influencing the presentation and management of hair disorders like alopecia areata.

Dermatology Times is recapping our exclusive expert interviews from the month of November.
Michael Lewitt, MD, reassures patients that topical ruxolitinib shows minimal systemic absorption despite sharing a mechanism with oral JAK inhibitors.

High-dose topical delgocitinib significantly outperformed oral alitretinoin in reducing HECSI severity at both 12 and 16 weeks.

Discover the potential of INF904, a groundbreaking oral therapy targeting C5aR for hidradenitis suppurativa and chronic spontaneous urticaria, promising new treatment options.

Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.