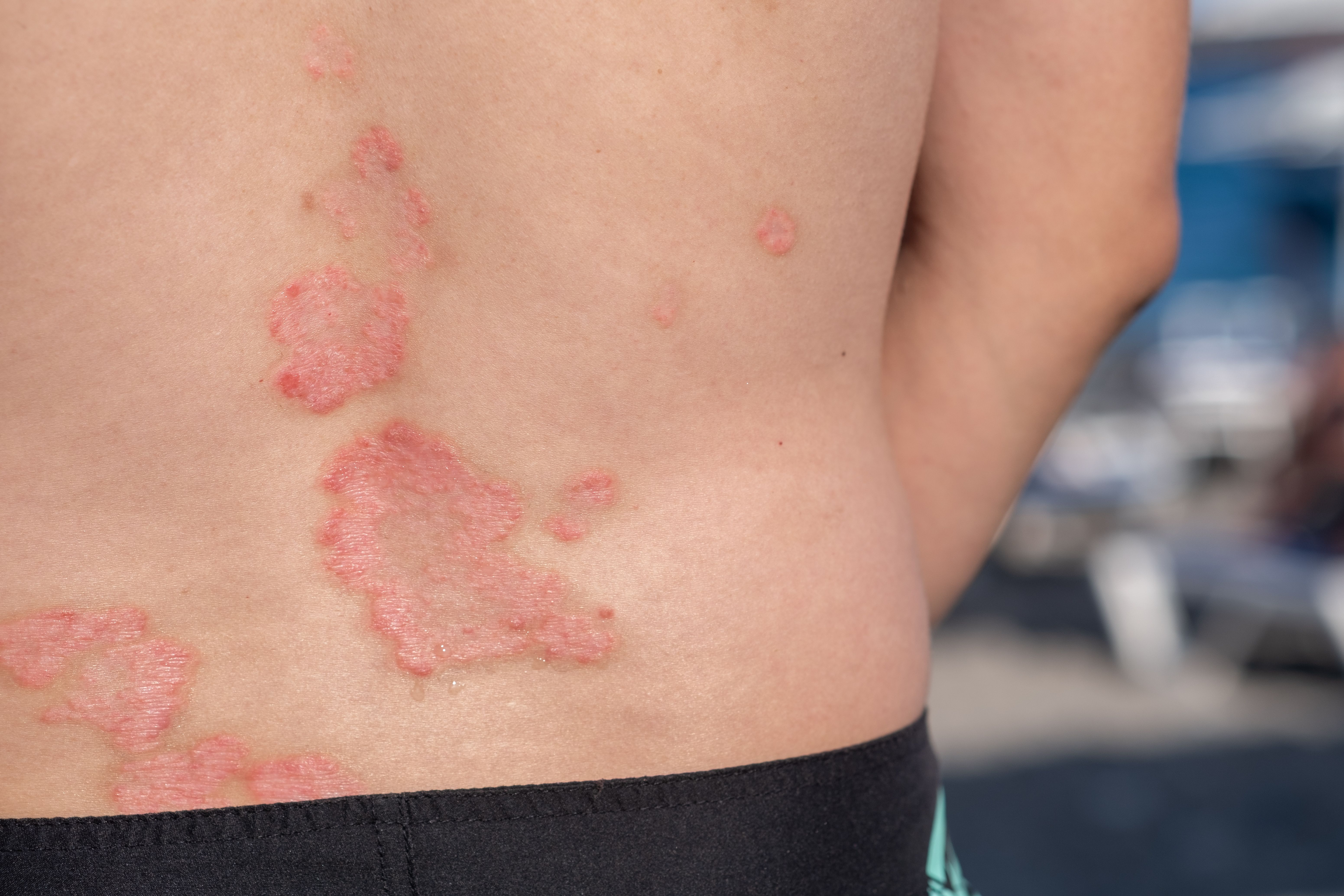
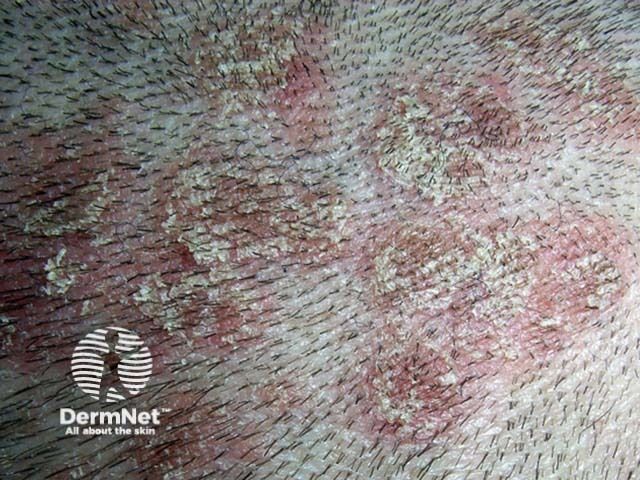
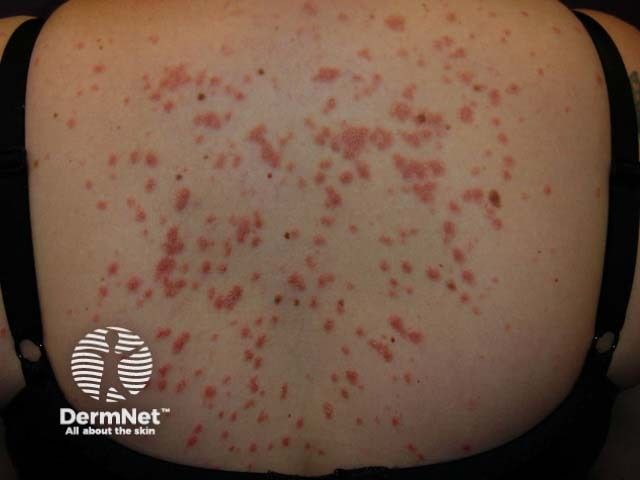
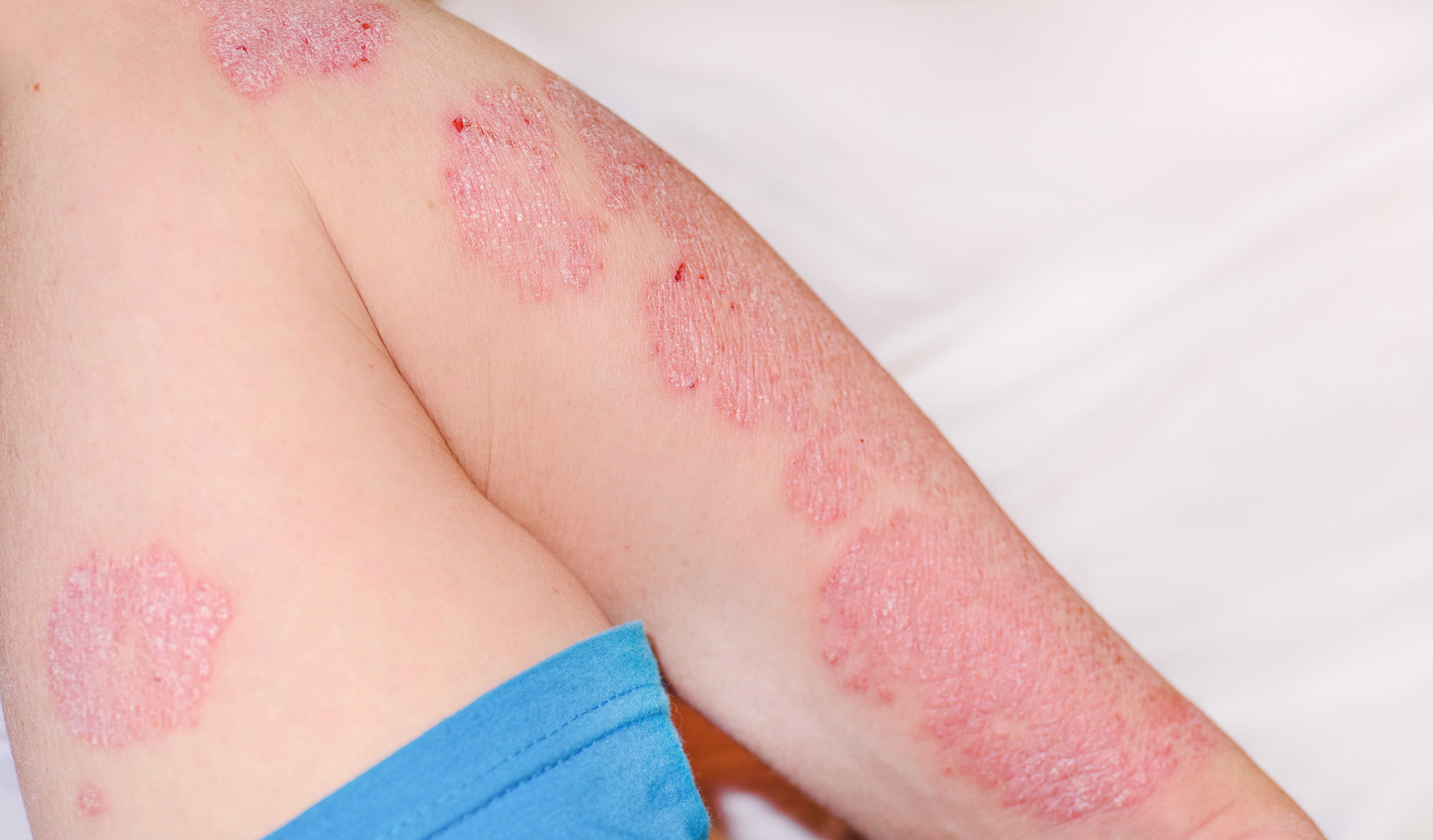
Psoriasis
Latest News
Video Series

Latest Videos
Shorts
CME Content
More News
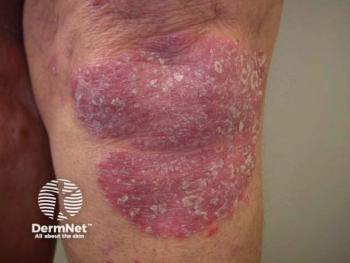
Oruka Therapeutics reveals promising interim results for ORKA-002 and launches the EVERLAST-B trial for ORKA-001, targeting chronic skin diseases.

Explore how precision dermatology leverages immune pathways to enhance treatment for inflammatory skin diseases.
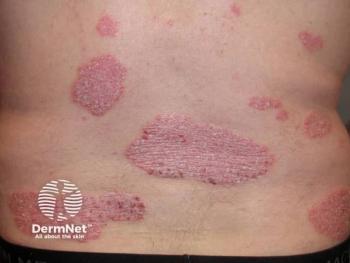
Envudeucitinib shows promising results as a new oral therapy for psoriasis, achieving high skin clearance rates and rapid symptom improvement in clinical trials.

Chronic skin diseases like psoriasis and AD are linked to high smoking and other addiction rates, highlighting the need for integrated psychosocial support.

Explore the latest French guidelines for systemic psoriasis therapy, highlighting new treatments and tailored management strategies for diverse patient needs.
Discover the latest advancements in psoriasis treatment, including innovative therapies and personalized care options for improved patient outcomes in 2025.

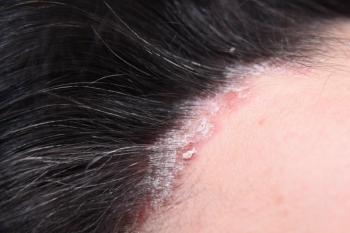
In part 3 of this Case-Based Roundtable supplement, experts discuss psoriatic therapies for hair-baring areas.

Omar Noor, MD, recently presented 3 cases of psoriasis in older patients at Horizons in Advanced Practice to discuss available treatment options and considerations for Medicare and in-office injections.
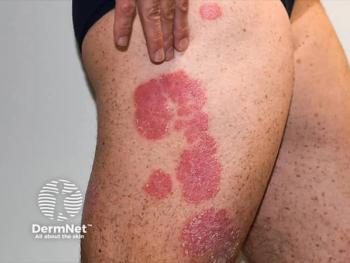
Zasocitinib emerges as a promising oral therapy for psoriasis, achieving significant skin clearance and favorable safety in recent phase 3 trials.
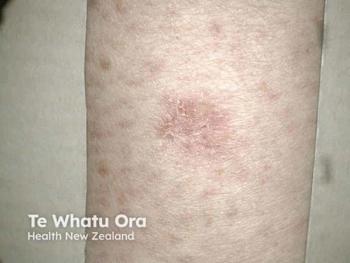
Soligenix reveals promising results from its phase 2a study of SGX302, a novel therapy for mild-to-moderate psoriasis, enhancing patient experience and efficacy.
Discover the latest breakthroughs in atopic dermatitis and psoriasis treatments for 2025, enhancing patient care and individualizing therapy options.

At the Horizons in Advanced Practice meeting, Douglas DiRuggiero, DMSc, MHS, PA-C, discussed psoriasis in high-impact sites, overall psoriatic disease, and available therapies.

In part 2 of this Case-Based Roundtable supplement, experts discuss therapeutic options for psoriasis when considering involvement of special areas.

In part 1 of this Case-Based Roundtable supplement, experts discuss biologic treatment options for psoriasis and balancing efficacy with patient needs.
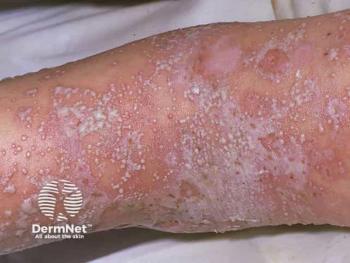
The BLA is supported by phase 3 data demonstrating rapid skin clearance and sustained disease control in patients with GPP.

A meta-analysis of over 800,000 patients shows JAK inhibitors are generally as safe as TNF antagonists for various purposes.

Discover the efficacy and safety of topical roflumilast 0.3% for psoriasis, highlighting its rapid results and potential for sensitive skin areas.

A study reveals high rates of intimate partner violence among women with psoriasis, linking it to poorer quality of life and increased PTSD symptoms.
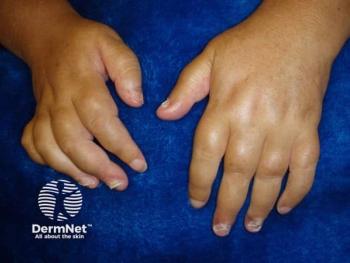
New long-term findings reveal guselkumab (TREMFYA) as the only IL-23 inhibitor effectively preventing joint damage in active psoriatic arthritis.

A Prescription Drug User Fee Act target action date is set for June 29, 2026.

The exclusive event brought together physician assistants and nurse practitioners from across the country to review complex atopic dermatitis, psoriasis, chronic hand eczema, and hidradenitis suppurativa patient cases.

Karan Lal, DO, explores the transformative role of GLP-1 therapies in dermatology, highlighting their potential in treating inflammatory skin diseases and enhancing aesthetic care.

Experts discuss icotrokinra's potential to revolutionize psoriasis treatment with oral therapies, emphasizing efficacy, safety, and patient-centered care at EADV 2025.

At a Case-Based Roundtable event, Cory Rubin, MD, discussed real-world strategies for optimizing systemic therapy in patients with moderate to severe psoriasis.
ORKA-001 by Oruka Therapeutics offers promising yearly dosing for psoriasis, showcasing extended efficacy and safety in early clinical trials.