Skin Cancer
Latest News
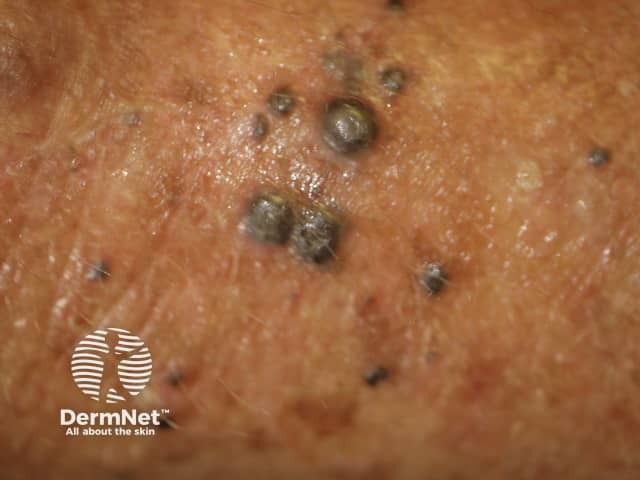
Latest Videos

CME Content
More News

Research reveals that structural changes in neoantigens enhance tumor rejection, offering new insights for personalized cancer immunotherapies.
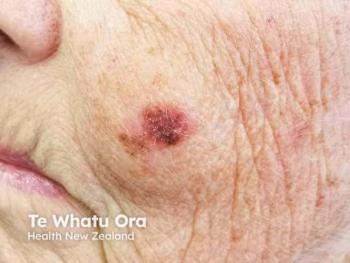
Elderly populations are facing rising cutaneous malignant melanoma incidence and mortality, highlighting urgent needs for targeted prevention and early detection strategies in skin cancer care.

New research reveals that combining DecisionDx-UM and PRAME significantly enhances survival predictions for uveal melanoma.
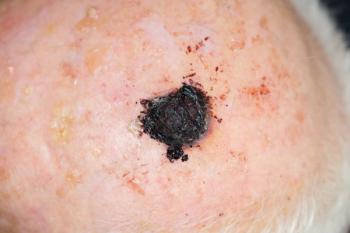
An expert panel endorses Castle Biosciences’ 31-GEP test, enhancing melanoma management through personalized risk assessment and improved prognostic accuracy.
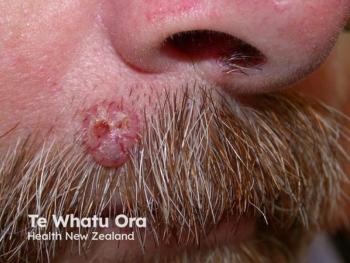
Topline results are expected in the first quarter of 2026.
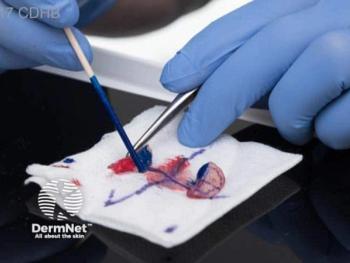
Former US President Joseph R. Biden's Mohs surgery highlights the need for equitable access to this effective skin cancer treatment, emphasizing its benefits and ongoing disparities.
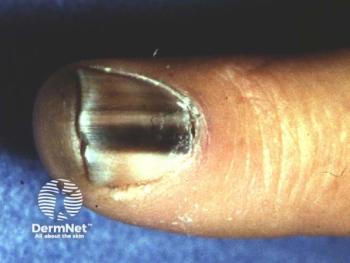
Alpha-9 Oncology's A9-3408 trial targets the melanocortin 1 receptor (MC1R) in advanced melanoma, offering a novel radiotherapeutic approach beyond traditional treatments.

New findings showcase DecisionDx-Melanoma's effectiveness in predicting sentinel lymph node positivity and enhancing recurrence risk assessment in melanoma patients.
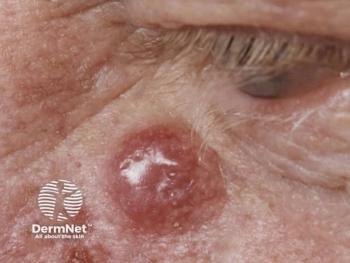
Researchers are developing an innovative mRNA vaccine targeting Merkel cell carcinoma, enhancing T cell immunity and offering new hope for effective treatment.

Renata Block, DMSc, MMS, PA-C, explores how AI could enhance dermatology, improving skin cancer diagnosis while emphasizing the vital role of human clinicians in patient care.

Findings suggest immune dysregulation and chronic inflammation in atopic dermatitis may contribute to increased skin cancer susceptibility.

An AI-powered handheld spectroscopy device is showing promise in improving melanoma detection accuracy among primary care physicians, addressing critical gaps in early diagnosis and timely referral.

Phio Pharmaceuticals reveals promising results for PH-762, a novel siRNA therapy targeting skin cancer, showcasing effective tumor clearance and safety.
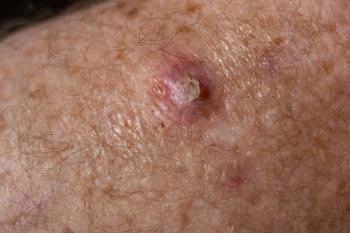
Medicus Pharma has initiated a phase 2 trial in the United Arab Emirates (UAE), exploring noninvasive treatment for basal cell carcinoma (BCC) using innovative microneedle technology.

The resubmission includes additional data and analyses addressing prior FDA concerns and is now classified as a complete response under a Class II review.

The CHMP opinion follows recent US FDA approval, positioning Libtayo as the first immunotherapy approved for adjuvant treatment in high-risk CSCC.
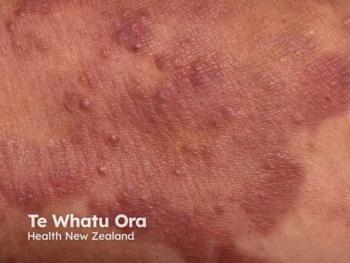
A recent case reinforced excimer laser therapy as a precise, efficient, and well-tolerated approach in managing cutaneous T-cell lymphoma variants.

Regeneron's cemiplimab-rwlc gains FDA approval as the first immunotherapy for high-risk cutaneous squamous cell carcinoma, significantly improving disease-free survival.
The vaccine is designed to stimulate T cell responses against IDO1- and PD-L1–positive cells within the tumor microenvironment.
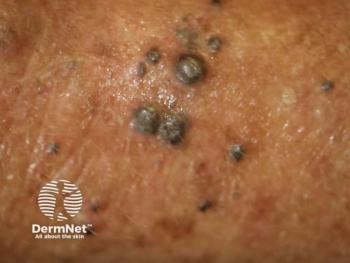
A new hybrid model combining deep learning with clinical data achieved high accuracy in predicting sentinel lymph node metastasis in melanoma.

Thazin Aung, PhD, discusses AI's role in standardizing immune cell evaluation in melanoma, enhancing diagnostic consistency and clinical decision-making.

Medicus Pharma has initiated a phase 2 trial in the UAE for a non-invasive BCC treatment, aiming to revolutionize skin cancer care.

Greater awareness of UV risks and stricter sun protection have contributed to declining invasive melanoma rates.

COVID-19 significantly delayed melanoma diagnoses and treatments, leading to increased disease severity and advanced cases, highlighting urgent health care needs.