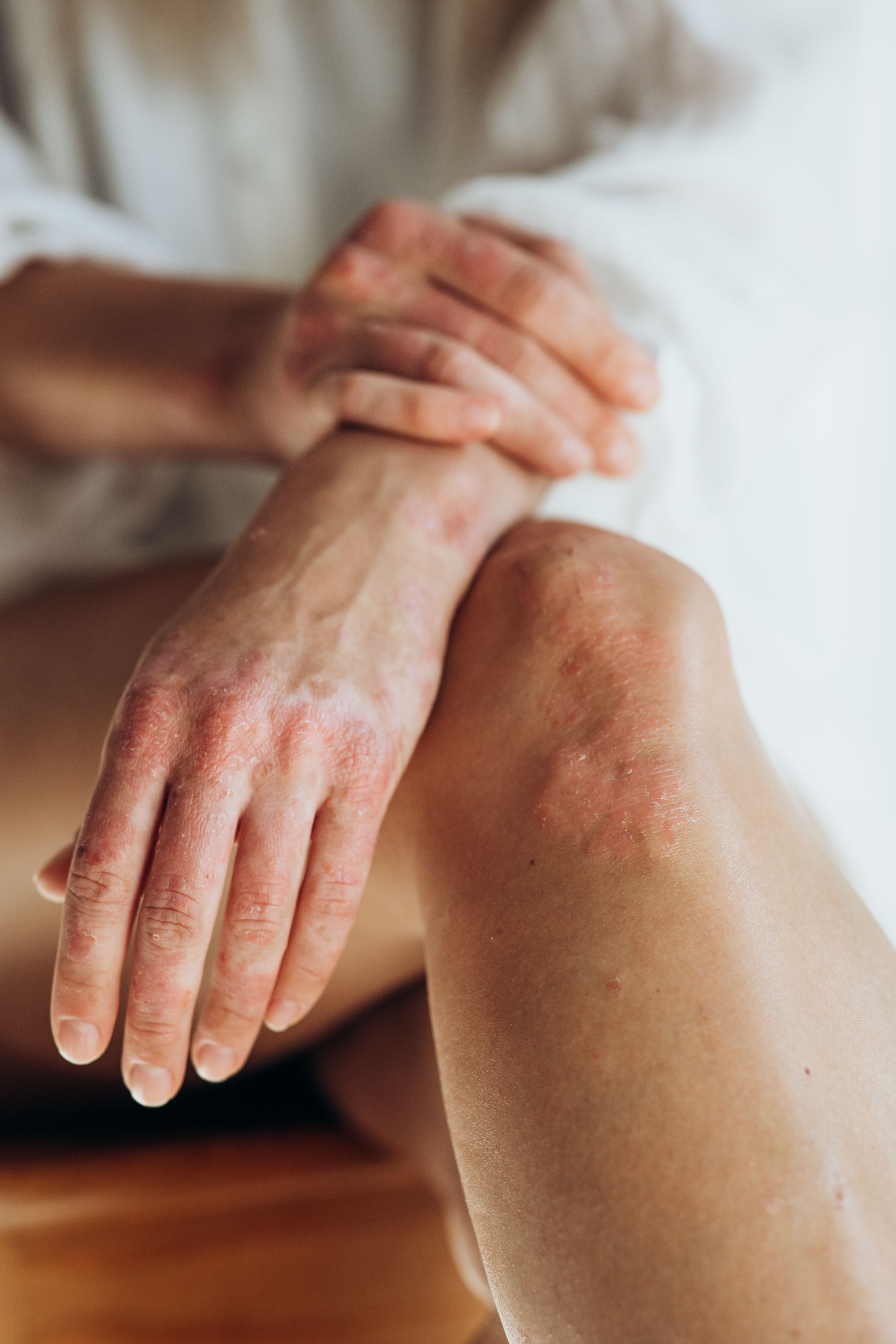
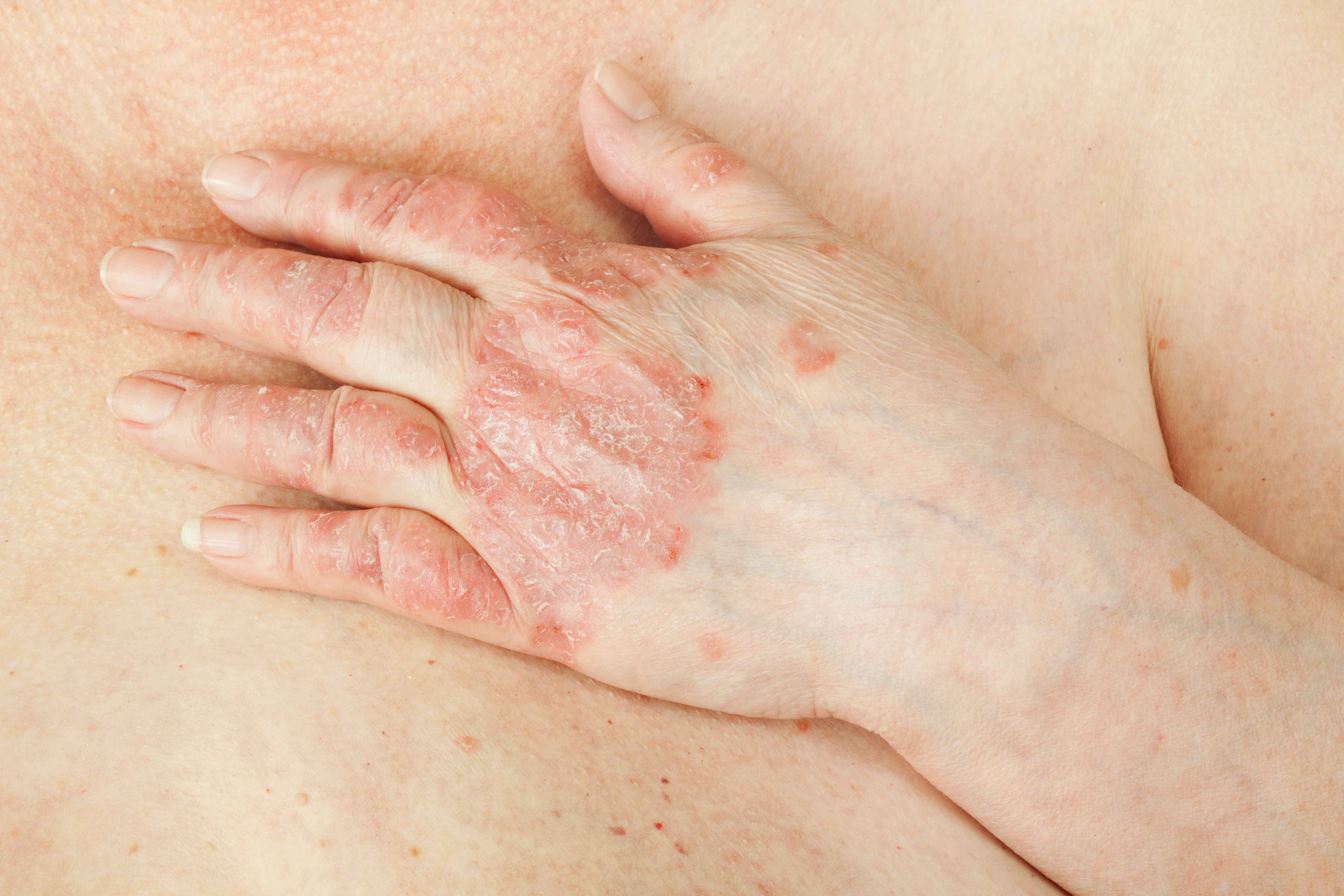
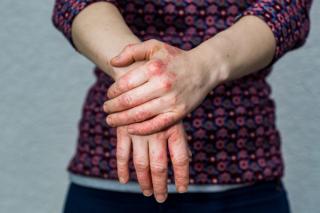
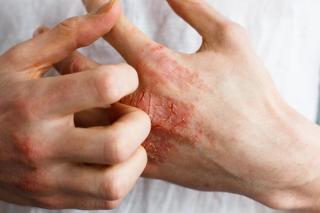
Psoriatic Arthritis
Latest News
Latest Videos

CME Content
More News
By blocking 2 key inflammation signals, research presented at EADV found bimekizumab may help keep both skin and joints healthier over time.
Deucravacitinib-treated patients achieved superior clinical responses compared to placebo at week 16 in both trials.

Johnson & Johnson seeks FDA approval to update guselkumab's label, highlighting its unique ability to significantly inhibit joint damage in active psoriatic arthritis.

Sun Pharma reveals promising phase 3 results for tildrakizumab (Ilumya) in treating PsA, showing significant symptom improvement in patients.
Bristol Myers Squibb's deucravacitinib gains global regulatory acceptance for treating active psoriatic arthritis, promising a new oral treatment option.

Apremilast improved joint symptoms and quality of life in patients with early oligoarticular PsA, with consistent efficacy across all BMI categories.

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!

Data presented at EULAR 2025 showcased Tremfya's efficacy as the only IL-23 inhibitor to demonstrate inhibition of this nature in psoriatic arthritis.

At the 2025 EULAR meeting, UCB presented new 3-year data on bimekizumab for PsA.

The National Psoriasis Foundation Seal of Recognition program has expanded to prescription drugs, making roflumilast the first to achieve the indication.

Tremfya is the first IL-23 inhibitor to significantly improve both symptoms and structural damage in active psoriatic arthritis.

Unlike biologics, nonbiologic systemic therapies showed a neutral or slightly increased cardiovascular risk, raising concerns about their long-term safety.

A study reveals that biologics targeting IL-12, IL-23, and IL-17 lower the risk of serious infections in older adults with psoriatic disease.

According to a poster from AAD, the National Psoriasis Foundation’s treatment goals were used as a benchmark to determine the risk of developing psoriatic arthritis after initiating biologics.

Ustekinumab-kfce is available for the treatment of chronic autoimmune conditions like Crohn’s disease, ulcerative colitis, and plaque psoriasis.

Advances in biologics, JAK inhibitors, and PDE4 inhibitors provide personalized options for psoriasis management.

Researchers found that PsA patients prioritize fatigue, sleep issues, and uncertainty, often ranking them higher than doctors do.

Effective PsA management requires balancing clinical goals with patient-reported challenges like fatigue and daily disruptions.

Deucravacitinib achieved ACR20 response in patients with PsA at week 16, with a safety profile consistent with previous studies.

The FDA recently approved Yesintek for treating psoriasis, psoriatic arthritis, and IBD, with a 2025 launch expected.

2-year data show 75.7% of TNFi-IR patients maintained ACR50, 80.6% achieved PASI100, and pain and fatigue improvements were sustained.

The new delivery systems for bimekizumab-bkzx represent an upgrade over previous 1 mL options, reducing the frequency of injections required.

The study design emphasizes elevated endpoints, particularly joint health, ensuring that the most relevant outcomes are prioritized for patient care.

Otulfi from Formycon and Fresenius Kabi was approved simultaneously in the US and the European Union. In the US, it will launch in February 2025.

Mease, the lead investigator on the trial, spoke about comparing varying mechanisms of action, primary and secondary endpoints, and how the results of BE BOLD could affect treatment choices.