Secukinumab Receives FDA Approval for Pediatric Patients with PsA
Secukinumab is the only biologic treatment in the US that’s approved by the FDA for pediatric patients with enthesitis-related arthritis (ERA) and psoriatic arthritis (PsA).
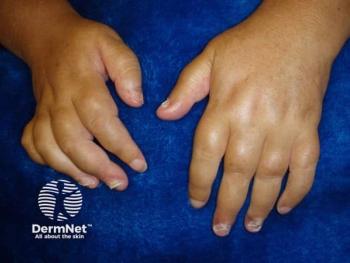
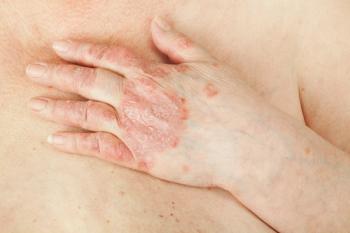
Novartis today announced the FDA has approved secukinumab (Cosentyx; Novartis) for the treatment of active enthesitis-related arthritis (ERA) in patients 4 years and older, and active psoriatic arthritis (PsA) in patients 2 years and older.1
This makes secukinumab is now the first biologic indicated for ERA and the only biologic treatment approved for both ERA and PsA in pediatric patients in the US. Also, these are the second and third approvals for secukinumab in a pediatric population in the US, and it now has a total of five indications across rheumatology and dermatology.1
"Prior research suggests that despite receiving treatment, some children and adolescents with PsA or ERA can continue to experience symptoms," said Hermine Brunner, MD, Cincinnati Children's Hospital. "The findings from the Phase III JUNIPERA trial show that pediatric patients treated with secukinumab demonstrated marked responses throughout the treatment period. This approval is positive news for some patients who continue to struggle with painful symptoms like inflammation of the joints and swollen fingers and toes."
The approved pediatric dosing for secukinumab in children and adolescents is 75 mg (body weight: 15 kg to less than 50 kg) or 150 mg (50 kg or more)
"The FDA approvals for JPsA and ERA follow the approval of Cosentyx as a first-line systemic treatment for pediatric psoriasis earlier this year, and further reinforce the commitment of Novartis to the pediatric community," said Victor Bulto, Head, US Pharmaceuticals, Novartis Pharmaceuticals Corporation. "Cosentyx is a proven medicine with a history of efficacy and safety across several systemic inflammatory conditions, with more than 500,000 patients treated worldwide since launch."
The approval is based on data from the Phase 3 JUNIPERA (NCT03031782) study. It is a 2-year, three-part, double-blind, placebo-controlled, randomized-withdrawal trial that enrolled 86 pediatric patients aged 2 to 17 years with a confirmed diagnosis of ERA or JPsA according to a modified International League of Associations for Rheumatology classification criteria.
The primary endpoint of the study was time to flare in the treatment period 2 (Week 12 to Week 104). It was demonstrated that patients with active JPsA (n = 34; mean age: 12.2) treated with secukinumab longer time to flare, showing a 85% reduction in the risk of flare (P<.001) versus placebo, according to the release. The study also demonstrated that patients with active ERA (n = 52; mean age: 13.7) treated with secukinumab had a longer time to flare, showing a 53% reduction in the risk of flare versus placebo. Safety in this pediatric population was consistent with the known safety profile of secukinumab for the treatment of plaque psoriasis, PsA, non-radiographic axial spondyloarthritis and ankylosing spondylitis.
"The symptoms of PsA and ERA can be debilitating for children and adolescents living with these chronic conditions, impacting their daily lives," said Tiffany Westrich-Robertson, CEO, International Foundation for Autoimmune & Autoinflammatory Arthritis (AiArthritis). "It is encouraging to see an additional treatment option for these underserved patient populations."
Reference:
1. Novartis Cosentyx receives FDA approval for the treatment of children and adolescents with enthesitis-related arthritis and psoriatic arthritis. Accessed December 23, 2021. https://www.prnewswire.com/news-releases/novartis-cosentyx-receives-fda-approval-for-the-treatment-of-children-and-adolescents-with-enthesitis-related-arthritis-and-psoriatic-arthritis-301450193.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.