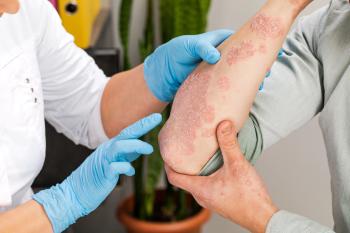
Posters at AMCP Nexus analyzed the burden of flares on patients with atopic dermatitis (AD) and the impact initiating ruxolitinib cream had on use of other therapies.

Posters at AMCP Nexus analyzed the burden of flares on patients with atopic dermatitis (AD) and the impact initiating ruxolitinib cream had on use of other therapies.

Otulfi from Formycon and Fresenius Kabi was approved simultaneously in the US and the European Union. In the US, it will launch in February 2025.

The rates of discontinuation due to inefficacy or adverse events for biosimilars and originators of etanercept and adalimumab were similar, according to an analysis of a prospective registry.

A matching-adjusted indirect comparison found that some patients with PsA are more likely to achieve long-term treatment outcomes with bimekizumab.

Up to one quarter of patients with PsA experience persistent joint pain. In a recent study, higher levels of fatigue, depression, and anxiety were associated with joint pain.

While the first ustekinumab biosimilar, Wezlana, was approved in October 2023, a settlement with Johnson & Johnson (J&J) will keep it off the market until 2025, preventing competition, and causing purchasers to pay substantially more for the agent.

Research has shown that biosimilars are highly similar to their originator product in terms of safety and efficacy.

A real-world study of children with moderate-to-severe atopic dermatitis (AD) in China found Dupixent can reduce symptoms and improve pruritus. Traditional therapies had little effect, according to the researchers.

A national study in Germany identified the baseline characteristics of patients with moderate-to-severe atopic dermatitis (AD) to understand how they are usually treated with Dupixent in the real world.

The increases had no impact on the efficacy of the drug and were rarely associated with symptoms or sequelae.