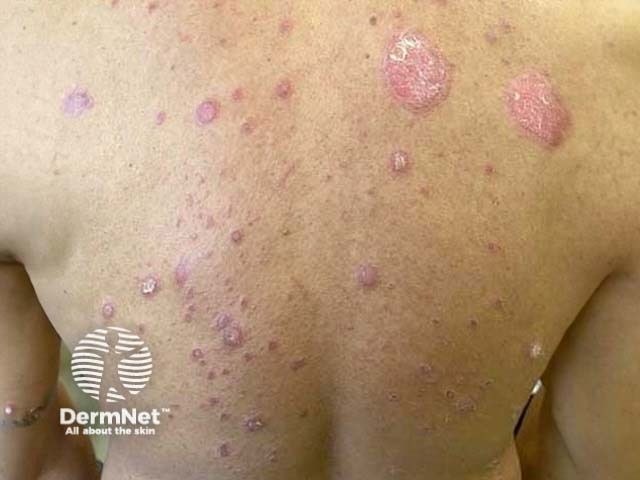
Psoriasis
Latest News

Latest Videos

CME Content
More News

Omar Noor, MD, FAAD, hosted a live Case-Based Roundtable discussion with local dermatology clinicians to discuss 2 patients with plaque psoriasis and what would be the ideal topical treatment for each.

Researchers behind the study emphasized the urgent need to enhance the diagnostic skills of primary care practitioners to improve early detection of psoriasis.

Otulfi from Formycon and Fresenius Kabi was approved simultaneously in the US and the European Union. In the US, it will launch in February 2025.

The IL-17 inhibitor demonstrated biologic-level efficacy in moderate to severe plaque psoriasis.

Over 70% of patients achieved clear or minimal skin by week 48, with a 90% retention rate in diverse patients with PsO.

Melinda Gooderham, MSc, MD, FRCPC, emphasized the significance of the study’s results, showcasing stable laboratory parameters over a 12-week trial.
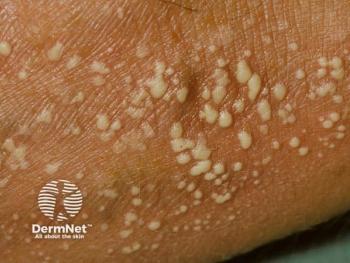
While researchers found both therapies effective in long-term use, they stated secukinumab showed quicker improvements in QoL.

Enhanced education can reduce uncertainty among both patients and providers, with pharmacists playing a key role as primary sources of patient information.

This marks the first head-to-head trial of an IL-17A/F inhibitor against an IL-23 inhibitor in psoriatic arthritis treatment.

The company plans to release full 52-week OLE data for ESK-001 in early 2025, building on positive interim results.
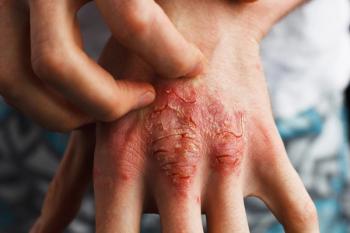
The findings stem from post-hoc analyses of previous trials, which found that 75.1% of patients reached PASI100 after 1 year on bimekizumab.

Through collaboration with Nimbus Therapeutics and Schrodinger, zasocitinib’s AI-driven design maximizes its fit within the targeted enzyme.

Mona Shahriari, MD, FAAD, shared insights into prevalence, clinical presentations, increasing research diversity, and advocating for representation.
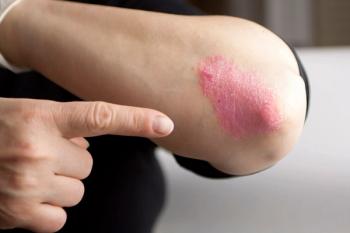
Researchers presented interim 3-year results from the European SERENA study.
Christopher Bunick, MD, PhD, shared insights into zasocitinib’s presented data and ongoing clinical trials.

The FDA has set a PDUFA target action date of May 22, 2025.

Zelma Chiesa Fuxench, MD, MSCE, FAAD, and Mona Shahriari, MD, FAAD, discuss the lack of research into psoriasis in skin of color, the absence of diversity in clinical trial populations, and where dermatology as a discipline can go from here.

Dermavant’s sNDA for tapinarof cream 1% for AD is still under review with the FDA.

Hawkes highlights groundbreaking therapies for psoriasis, including new oral medications and IL-23 inhibitors.

At Maui Derm NP+PA Fall 2024, Melodie Young, MSN, ANP-c, reviews new treatment options for pediatric patients with psoriasis and details the difference in disease manifestation between adults and children.
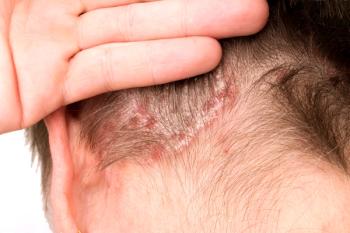
Armstrong shared insights on the recently released NPF psoriasis health indicator report including pearls on national health data and unmet needs in public health campaigns.
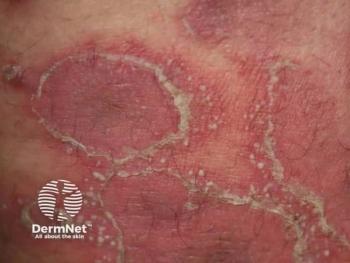
Researchers authored a manuscript detailing considerations for GPP diagnosis, definitions, and management.

The trial showcased the continued efficacy of apremilast, with 2-year data on the forefront.

The study reported that UST leads in dose escalation frequency, but also had lower discontinuation rates when compared to ADA and ETN.
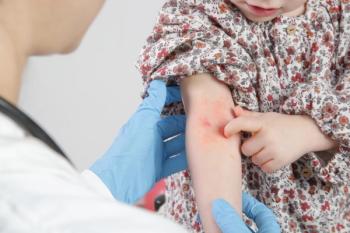
Dermatology Times spoke with Leah Howard, JD, of the NPF, and Trisha, the mother of a young girl with psoriasis, to discuss Otezla's implications in the therapeutic landscape.