Clear Communication Helps Reduce Patient Concerns About Boxed Warnings
Michael Lewitt, MD, reassures patients that topical ruxolitinib shows minimal systemic absorption despite sharing a mechanism with oral JAK inhibitors.
Episodes in this series

In an interview with Dermatology Times, Chicago-based dermatologist and clinical investigator Michael Lewitt, MD, discussed the clinical considerations surrounding the boxed warning for topical ruxolitinib and the broader landscape of evolving dermatologic therapies. Lewitt, a partner at Illinois Dermatology Institute and clinical researcher with Denova Research, emphasized the importance of historical context and careful patient communication when addressing safety concerns associated with Janus kinase (JAK) inhibitors.
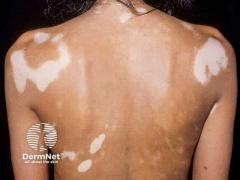
Lewitt notes that the boxed warning for topical ruxolitinib often triggers patient concern, but its origin lies largely in data from oral JAK inhibitor use rather than topical formulations. As he explained, “Two of the off-label drugs we used for many years to treat vitiligo, tacrolimus and pimecrolimus, people often forget about the box warning.” Those medications have carried boxed warnings for over 2 decades, giving clinicians extensive experience in framing risk in a patient-friendly manner.
The current warning stems from the FDA’s evaluation of tofacitinib safety data in rheumatoid arthritis. Lewitt summarized the findings for patients as follows: “When a drug with a similar mechanism acts, orally, plus another immunosuppressive drug…were administered together for a different disease state…we saw a higher risk of X, Y and Z.” These risks included malignancy, major adverse cardiovascular events (MACE), thromboembolic events, and overall mortality in older, cardiovascular-risk–enriched patients. Because of mechanistic similarities across JAK inhibitors, the FDA applied class-wide boxed warnings, even to topical agents with minimal systemic absorption.
Lewitt emphasizes to patients that “I am comfortable giving this to you or your child because I know it's not going to be absorbed. I just want you to know it’s there.” He stresses transparency but also reassures that topical ruxolitinib has not shown meaningful systemic exposure in published studies.
Beyond safety communication, Lewitt discussed the expanding therapeutic toolkit in dermatology. Early in his career, he recalls feeling limited in treatment options, stating, “I felt like my tool belt was a little bit light…here are three bricks and one wire.” Today, he notes a robust pipeline of targeted, nonsteroidal agents for conditions including atopic dermatitis, psoriasis, and vitiligo, many with approval down to age 2.
He expressed particular enthusiasm for future combination approaches, including potential pairing of topical agents with systemic therapies to optimize control and reduce therapeutic switching. This concept, he says, may help patients maintain disease stability more efficiently and reduce overall health care costs. Studies investigating these combinations will be essential, and Lewitt anticipates that, “being able to use these targeted topicals for touch-up paint and combination therapy but be able to prove that in studies, I think would be really neat.”
Lewitt concludes that dermatology is entering an era of increasingly precise, multimodal treatment strategies. Rigorous evidence will be needed to validate combination therapy approaches, but he views the future as promising for both clinicians and patients navigating chronic inflammatory skin diseases.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.