Lewitt Highlights Early Treatment Benefits of Topical JAK Inhibitors
Key Takeaways
- Topical JAK inhibitors show similar efficacy and tolerability in adults and adolescents, with minimal adverse effects and no significant systemic absorption.
- Adherence is critical for treatment success, with a focus on patient-centered approaches and education, especially for adolescents.
Michael Lewitt, MD, emphasizes adherence to twice-daily application as the primary determinant of clinical success.
Episodes in this series

In a recent discussion with Dermatology Times, Michael Lewitt, MD, partner at the Illinois Dermatology Institute and clinical investigator with Denova Research, provided detailed insight into the evolving role of topical Janus kinase (JAK) inhibitors, including ruxolitinib, in dermatologic practice. His comments reflect both clinical trial experience and real-world patient management across diverse populations.
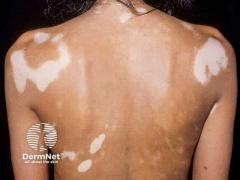
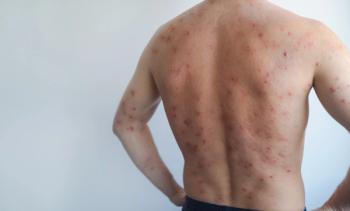
Lewitt emphasized that treatment outcomes in adults and adolescents using topical JAK inhibitors are fundamentally similar when medication is used as intended. As he noted, “The earlier we treat, the better the outcomes we are going to get,” highlighting the importance of timely intervention in inflammatory dermatologic conditions such as atopic dermatitis and vitiligo.
Tolerability of topical JAK inhibitors appears favorable across age groups. Lewitt stated that “Adults and adolescents tolerate the medicine both quite well,” with minimal adverse effects reported outside of occasional localized acne. Maximal-use studies, he explained, reinforce the safety profile: “We’re not getting systemic absorption of this drug…at least at quantifiable or dangerous levels.” This has supported increased clinician confidence in prescribing these therapies for both short- and long-term disease management.
A recurring theme in Lewitt’s comments was the central role of adherence. He prefers the term “adherence” rather than “compliance,” noting its more patient-centered framing. Achieving positive outcomes, he said, “all kind of boils down to…really adhere and be able to get that twice-daily application.” Engagement with adolescent patients may require additional education, but overall response patterns remain consistent with adults when medication is used correctly.
Beyond clinical considerations, Lewitt addressed the structural barriers that often limit access to advanced topical therapeutics. He acknowledged the challenges posed by high wholesale acquisition costs and insurance requirements, particularly for underserved communities and patients with skin of color. To navigate these obstacles, Lewitt relies heavily on documentation, specialty pharmacies, and patient assistance pathways. “Usually where there's a will, there's a way,” he remarked, citing the role of trained staff, coupon programs, and manufacturer-supported pharmacies in reducing patient out-of-pocket burden.
Clinical trials remain an important avenue for patients who cannot obtain approved therapies. Lewitt encourages trial participation when appropriate, emphasizing ethical safeguards and the presence of active treatment arms. He often reassures patients that participation is not experimental in the traditional sense, particularly when a drug is already approved for another condition.
Lewitt also underscored the importance of representative clinical research. Diverse Fitzpatrick skin types included in recent trials allow clinicians to counsel patients using data reflective of their own skin type. This representation, he noted, helps patients “feel a lot more comfortable about trying something new.”
Overall, Lewitt’s insights portray topical JAK inhibitors as effective, well-tolerated therapeutic options whose success depends on adherence, thoughtful documentation, and proactive approaches to access and equity.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.