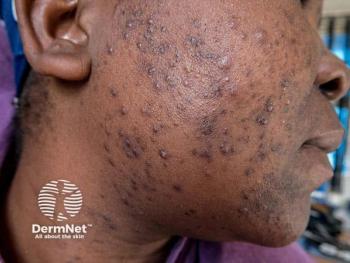
Discover how a novel 1550-nm laser treatment effectively addresses acne scarring in diverse skin types, enhancing safety and results for all.
Maddi Hebebrand is an associate editor of Dermatology Times and joined the MJH Life Sciences team in May 2024. She attended Baldwin Wallace University, studying Media Production and Film, and received her Masters in Digital Media from Ohio University. When she's not writing, Maddi loves to read, attend concerts and spend time with her family.

Discover how a novel 1550-nm laser treatment effectively addresses acne scarring in diverse skin types, enhancing safety and results for all.

Explore the latest French guidelines for systemic psoriasis therapy, highlighting new treatments and tailored management strategies for diverse patient needs.
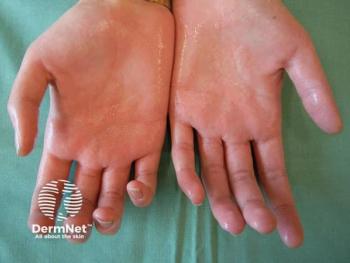
Explore the latest insights on hyperhidrosis, its impact on quality of life, and advancements in effective treatment options for this often-overlooked condition.

Explore the latest advancements in dermatology for 2026, featuring innovative therapies and treatments for skin conditions, hair loss, and more.

Explore the top headlines of 2025, including insights on the latest clinical trials, therapeutic updates, and more.

Discover groundbreaking FDA dermatology approvals in 2025, featuring innovative therapies for chronic skin conditions and pediatric care advancements.

Dermatology evolves in 2025, focusing on personalized care, safety, and technology integration, while addressing systemic health and patient outcomes.

Explore the top headlines of the month, including insights on regulatory updates, expert pearls, and more.
Discover the latest advancements in psoriasis treatment, including innovative therapies and personalized care options for improved patient outcomes in 2025.
Discover the latest advancements in CSU treatment, including new therapies and evolving management strategies for better patient outcomes.

Pediatric dermatology experiences a transformative shift, enhancing care for younger patients and expanding therapeutic options.
Discover the latest advancements in hidradenitis suppurativa therapies, including promising biologics and oral treatments reshaping patient care in 2025.

Erik Domingues leads a vital discussion on innovative vitiligo management, addressing treatment challenges and psychosocial impacts in diverse patient populations.
Michael O'Donoghue, MD, shares insights on actinic keratosis management, emphasizing personalized therapy strategies.


Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.
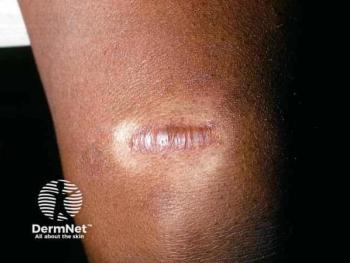
Discover effective treatment strategies for keloids and hypertrophic scars in skin of color, emphasizing personalized approaches and combination therapies.
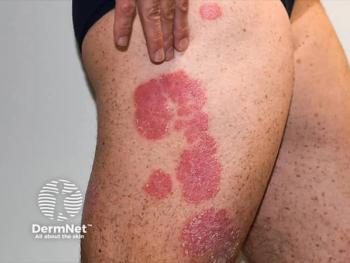
Zasocitinib emerges as a promising oral therapy for psoriasis, achieving significant skin clearance and favorable safety in recent phase 3 trials.

Porter leads a dynamic roundtable on hidradenitis suppurativa, exploring innovative treatments and collaborative strategies for improved patient outcomes.

New research reveals that combining DecisionDx-UM and PRAME significantly enhances survival predictions for uveal melanoma.

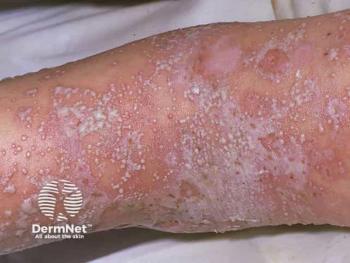
The BLA is supported by phase 3 data demonstrating rapid skin clearance and sustained disease control in patients with GPP.
Experts discuss innovative, steroid-sparing treatments for pediatric atopic dermatitis, emphasizing personalized care and proactive management strategies.

Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.

A meta-analysis of over 800,000 patients shows JAK inhibitors are generally as safe as TNF antagonists for various purposes.
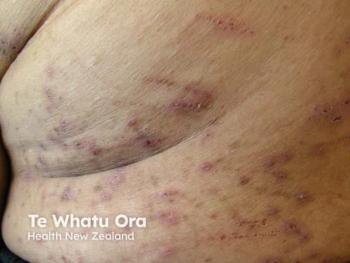
Galderma initiates a pivotal study on nemolizumab, targeting Chronic Pruritus of Unknown Origin, aiming to provide relief for this challenging condition.
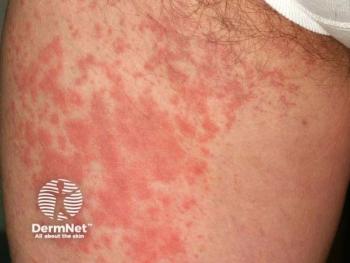
Explore the long-term efficacy and safety of ligelizumab for CSU, highlighting self-administration success and significant symptom control.
