
Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.

A study reveals high rates of intimate partner violence among women with psoriasis, linking it to poorer quality of life and increased PTSD symptoms.

At a Dermatology Times Case-Based Roundtable event, Joe Gorelick, MSN, FNP-C, reviewed real-world strategies for hidradenitis suppurativa, highlighting growing preference for bimekizumab based on its dual IL-17A/IL-17F inhibition.

Discover the latest insights on laser and energy-based treatments for rosacea, highlighting the effectiveness of radiofrequency microneedling and combination therapies.

Experts explore the integration of AI in atopic dermatitis management, highlighting benefits, risks, and the need for cautious implementation in patient care.
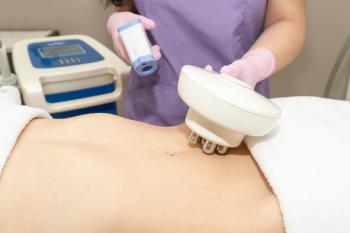
New study reveals how microneedle radiofrequency technology effectively addresses postpartum abdominal skin laxity, enhancing skin elasticity and patient satisfaction.

Selected pieces will be featured in Dermatology Times’ year-end and early-2026 coverage.
InnoCare Pharma has initiated a global trial for soficitinib, targeting prurigo nodularis, aiming to enhance treatment options for this chronic skin condition.
Omar Noor, MD, outlines the complex phenotypes, variable presentations, and functional burdens of chronic hand eczema and explains why a broader JAK-targeted strategy may offer therapeutic advantages.
Alpha-9 Oncology's A9-3408 trial targets the melanocortin 1 receptor (MC1R) in advanced melanoma, offering a novel radiotherapeutic approach beyond traditional treatments.

Dermatology Times has compiled a list of dermatological meetings taking place during the month of December.
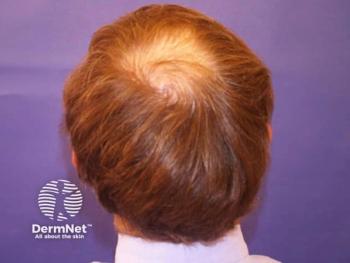
Cosmo Pharmaceuticals reveals promising phase 3 results for clascoterone 5% solution, showing significant hair growth improvement in alopecia.

Tori Spelling and Stella McDermott share their journey with eczema, promoting the Free to Be Me campaign and discussing innovative treatments like roflumilast.

At the Horizons in Advanced Practice meeting, Omar Noor, MD, led NP and PA attendees through 3 complex cases of atopic dermatitis, prurigo nodularis, and chronic spontaneous urticaria.

Arash Mostaghimi, MD, MPH, FAAD, and experts discuss evolving treatment standards for alopecia areata, focusing on JAK inhibitors, patient-centered care, and future therapeutic innovations.
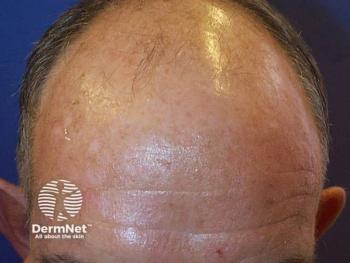
Discover how a proprietary extract from saw palmetto promotes hair growth and reduces thinning in adults, offering a safe alternative to traditional treatments.

The study provides new evidence beyond previously established associations with breast, ovarian, and uterine cancers.

Navigator Medicines advances NAV-240, a promising bispecific antibody for HS, targeting dual pathways for enhanced treatment efficacy.

This review of the latest dermatologic studies includes a new instrument to measure QoL in pediatric alopecia, BTX-A in patients with recessive dystrophic epidermolysis bullosa, eosinophilic pustular folliculitis in children, and more.
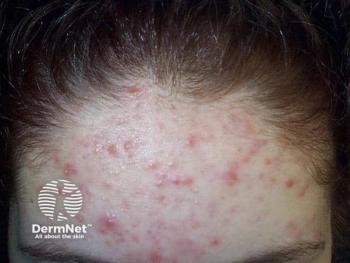
However, over half of the patients reported experiencing psychological symptoms such as mood changes and depression during treatment.
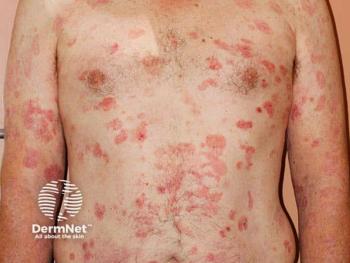
Citius Oncology’s IL-2 receptor–directed fusion protein offers rapid responses and pruritus improvement for relapsed or refractory stage I–III CTCL.

Participants agreed that HS is rarely controlled with monotherapy and often requires coordinated multimodal care.

Support vital dermatology organizations this Giving Tuesday and beyond to enhance patient care, drive research, and foster community.

Patrick Burnett, MD, PhD, of Arcutis, explores new treatments for vitiligo and hidradenitis suppurativa, while advancing systemic biologics for atopic dermatitis by 2026.

Mondana Ghias, MD, FAAD, offers strategies for dermatologists in managing skin reactions from lung cancer treatments to enhance patient care and quality of life.

While individual fatty acids in tallow have known biological effects, their combined impact in whole tallow remains uncertain.

Arcutis is advancing pediatric dermatology with innovative, steroid-sparing treatments for atopic dermatitis and plaque psoriasis, enhancing care for young patients.

Nayan Patel, PharmD, is revolutionizing skin rejuvenation with advanced delivery technology of GHK-Cu for youthful vitality.

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!