
Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

In case you missed it, this week we had news about LNK01004's phase 2 AD results, INF904's efficacy in HS and CSU, new data supporting tralokinumab for hand AD, and more.

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.

Explore the top headlines of the month, including insights on regulatory updates, expert pearls, and more.

Lynk Pharmaceuticals reveals promising phase 2 trial results for LNK01004, a topical treatment showing efficacy and safety in moderate-to-severe atopic dermatitis.

Dermatology Times is looking back on the top stories in dermatology from the month of November.
Discover the latest trends in aesthetic medicine from ASDS 2025, focusing on safer treatments, improved skin quality, and innovative injectables.
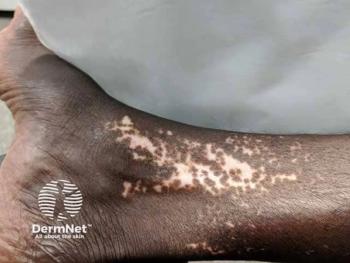
Hair growth, density, and aging patterns differ across populations, influencing the presentation and management of hair disorders like alopecia areata.

Learn more about the in-depth topics covered in the November 2025 print issue of Dermatology Times.

Discover the latest advancements in dermatology from the 2025 Elevate-Derm Fall Conference held in Tampa, Florida.

Dermatology Times is recapping our exclusive expert interviews from the month of November.

Find out what skin care products and aesthetic innovations clinicians are recommending to patients for the month of November.

At Elevate-Derm, Shanna Miranti, MPAS, PA-C, and Larissa Schmidt, DPM, outlined how interdisciplinary education, shared therapeutic advances, and evolving career pathways are strengthening the connection between dermatology and podiatry.

Experts reveal how neurotoxins enhance mature skin, emphasizing precision and lower doses for natural, youthful results.

This review of the latest dermatologic studies highlights new research from this week, including immune checkpoint inhibitors in acral melanoma, the link between skin microbiota and asthma, a novel peptide–hyaluronic acid lip serum, and more.

At Elevate-Derm 2025, Lisa Weiss, MMSc, PA-C, outlined the multifactorial approach to CHE, the clinical utility of delgocitinib, and her expanding educational and leadership priorities heading into 2026.
Michael Lewitt, MD, reassures patients that topical ruxolitinib shows minimal systemic absorption despite sharing a mechanism with oral JAK inhibitors.

Discover how Des Fernandes, MB. BCH, FRCS, revolutionized microneedling and regenerative skin care, enhancing results with topical vitamins for optimal skin health.

High-dose topical delgocitinib significantly outperformed oral alitretinoin in reducing HECSI severity at both 12 and 16 weeks.

James Canner, PA-C, outlines key updates in acne therapeutics, alopecia management, and rheumatology–dermatology collaboration presented at Elevate-Derm Fall.

LEO Pharma reveals promising 32-week results for tralokinumab in treating atopic dermatitis on hands, enhancing patient quality of life and safety.

Discover the potential of INF904, a groundbreaking oral therapy targeting C5aR for hidradenitis suppurativa and chronic spontaneous urticaria, promising new treatment options.
Discover the latest advancements in botulinum toxins, including TrenibotE's rapid effects and onabotulinumtoxinA's consistent results for aesthetic treatments.

Tono Health revolutionizes virtual dermatology by enhancing specialist access for HS and oncodermatology care, while prioritizing the doctor-patient relationship.

Discover 5 effective tax-saving strategies for investors in dermatology, enhancing portfolio management and planning for 2026 with OJM Group's expert insights.

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!

Explore the top headlines of the week, including insights on the latest clinical trials, therapeutic updates, and more.

In case you missed it, this week we had news about the phase 2/3 research on VDPHL01, new data on the DecisionDx-Melanoma GEP test, roflumilast's sNDA for pediatric plaque psoriasis, and more.

This week, we feature top articles from our sister publications on regulatory updates, clinical trial insights, and more.

Explore the latest advancements and expert insights from ISDS 2025 held in New York, New York.