
At Elevate-Derm, Shanna Miranti, MPAS, PA-C, and Larissa Schmidt, DPM, outlined how interdisciplinary education, shared therapeutic advances, and evolving career pathways are strengthening the connection between dermatology and podiatry.

Kaitlyn Bader is the assistant managing editor of Dermatology Times® and joined the MJH Life Sciences team in June 2022. She attended Otterbein University in Westerville, Ohio, and earned a Bachelor of Arts degree in literary studies with minors in creative writing and film studies. Prior to health care news, Kaitlyn worked in industrial marketing. She enjoys cooking, reading, and tending to her house plants.
You can reach her at kbader@mjhlifesciences.com.

At Elevate-Derm, Shanna Miranti, MPAS, PA-C, and Larissa Schmidt, DPM, outlined how interdisciplinary education, shared therapeutic advances, and evolving career pathways are strengthening the connection between dermatology and podiatry.

At Elevate-Derm 2025, Lisa Weiss, MMSc, PA-C, outlined the multifactorial approach to CHE, the clinical utility of delgocitinib, and her expanding educational and leadership priorities heading into 2026.

James Canner, PA-C, outlines key updates in acne therapeutics, alopecia management, and rheumatology–dermatology collaboration presented at Elevate-Derm Fall.

At Elevate-Derm Fall, Ken Korber, PA-C, MHPE, DFAAPA, highlighted how literacy-aware communication and team-based collaboration can elevate patient understanding, adherence, and outcomes.

At Elevate-Derm Fall, Ted Rosen, MD, detailed how rapidly evolving resistance in bacteria, fungi, viruses, and more is reshaping therapeutic choices.

At Elevate-Derm 2025, Diego Ruiz Dasilva, MD, emphasized cross-role teamwork and urged clinicians to consider positive JAK inhibitor safety data.

Stacey Swinehart, DNP, FNP-BC, DCNP, CNL, shares key takeaways for clinicians from the SDNP's recent webinar on detecting intimate partner violence among patients.

Catch up on coverage from the final day of the 2025 Elevate-Derm Fall Conference.

At Elevate-Derm, Buchi Neita, PA-C, discussed treatment expectations for vitiligo, triple-combination cream for acne, early diagnosis in HS, and what she’s looking forward to in the 2026 pipeline.

At Elevate-Derm Fall, Patricia Delgado, DNP, AGPCNP, DCNP, PMHNP, outlined practical ways dermatology clinicians can incorporate psychodermatology principles and previewed the upcoming Psych and Skin video series.

Lisa Weiss, MMSc, PA-C, and Justin Love, PA-C, discuss the importance of broad differentials, repeat biopsies, and national networking to strengthen diagnostic accuracy and provider support at Elevate-Derm Fall.

Catch up on coverage from the third day of the 2025 Elevate-Derm Fall Conference.

At Elevate-Derm Fall, Peter Lio, MD, discussed how flexible clinical reasoning and an expanding therapeutic pipeline, particularly hidradenitis suppurativa and atopic dermatitis, are reshaping patient management heading into 2026.

At Elevate-Derm Fall, sisters Shanna Miranti, MPAS, PA-C, and Larissa Schmidt, DPM, reviewed the benefits of cross-specialty collaboration for patient care.

Catch up on coverage from the second day of the 2025 Elevate-Derm Fall Conference.

At the Elevate-Derm Fall Conference, Peter Lio, MD, highlighted practical strategies for corticosteroid stewardship, the expanding role of nonsteroidal topicals, and emerging opportunities in early-age indications that are reshaping pediatric inflammatory dermatoses care.
At Elevate-Derm Fall, Victoria Garcia-Albea, NP, details the expanding educational landscape in hair disease management, the rise of dedicated hair health meetings, and practical pathways for clinicians looking to advance professionally through writing.

Catch up on coverage from the first day of the 2025 Elevate-Derm Fall Conference.

Victoria Garcia-Albea, NP, outlined evolving therapeutic strategies at Elevate-Derm Fall, highlighting low-dose oral minoxidil, off-label JAK inhibition for frontal fibrosing alopecia, Thiamidol in hyperpigmentation, and delgocitinib for chronic hand eczema.

Matthew Brunner, MHS, PA-C, discusses upcoming highlights of the 6th annual meeting for dermatology NPs and PAs.

Marc Serota, MD, discussed upcoming systemic JAK inhibitors, the value of ruxolitinib cream and phototherapy, and a personalized multimodal approach to improve repigmentation outcomes.

The exclusive event brought together physician assistants and nurse practitioners from across the country to review complex atopic dermatitis, psoriasis, chronic hand eczema, and hidradenitis suppurativa patient cases.

Marc Serota, MD, outlines how ruxolitinib cream supports gradual, sustained repigmentation, highlights the significance of “islands” of early pigment return, and discusses when to escalate or combine modalities to optimize outcomes.
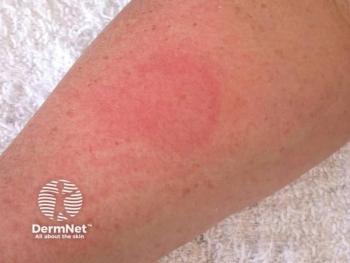
The first-in-class mast cell–selective c-Kit inhibitor ALY-301 enters clinical testing for cold urticaria in Germany.

At a Case-Based Roundtable event, Cory Rubin, MD, discussed real-world strategies for optimizing systemic therapy in patients with moderate to severe psoriasis.

Marc Serota, MD, discusses using ruxolitinib cream to safely maintain repigmentation, minimize steroid-related adverse effects, and enhance outcomes when combined with phototherapy.

An AI-powered handheld spectroscopy device is showing promise in improving melanoma detection accuracy among primary care physicians, addressing critical gaps in early diagnosis and timely referral.

The FDA issued 18 warning letters to websites illegally selling unapproved and misbranded botulinum toxin products, citing serious safety risks including botulism-related adverse events.

At the 10th Annual Symposium on Hidradenitis Suppurativa Advances, Ralph George, MD, FRCS, emphasized that the growing collaboration between medical and surgical management is redefining the role of procedures in HS.
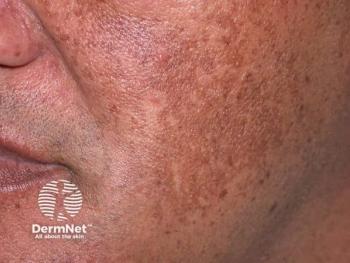
At Fall Clinical 2025, Andrew Alexis, MD, reviewed topical, oral, and procedural interventions with patients with melasma and hyperpigmentation, especially in patients with skin of color.