
Findings from a large retrospective study presented at EADV 2025 suggest psoriasis significantly increases the risk of both wet and dry age-related macular degeneration.

Kaitlyn Bader is the assistant managing editor of Dermatology Times® and joined the MJH Life Sciences team in June 2022. She attended Otterbein University in Westerville, Ohio, and earned a Bachelor of Arts degree in literary studies with minors in creative writing and film studies. Prior to health care news, Kaitlyn worked in industrial marketing. She enjoys cooking, reading, and tending to her house plants.
You can reach her at kbader@mjhlifesciences.com.

Findings from a large retrospective study presented at EADV 2025 suggest psoriasis significantly increases the risk of both wet and dry age-related macular degeneration.

Catch up on coverage from the first day of the 2025 European Academy of Dermatology and Venereology Congress held in Paris, France.

According to a poster at the 2025 EADV Congress, barzolvolimab achieved comparable improvements in weekly Urticaria Activity Scores across immunoglobulin E (lgE) subgroups.

At EADV 2025, UCB presented new long-term data demonstrating bimekizumab’s durable efficacy and a consistent safety profile over extended treatment.
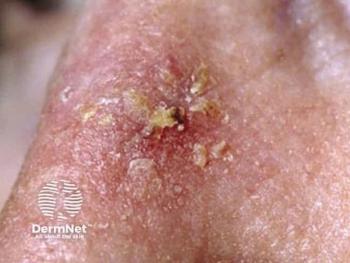
Biofrontera advances closer to expanding the Ameluz PDT label, as phase 3 follow-up data on actinic keratoses are expected next year.

Cigna’s removal of restrictions on home phototherapy, following a similar decision by Elevance Health, expands access to more than 66 million Americans.

In the latest Cutaneous Connection podcast episode, Renata Block, DMSc, MMS, PA-C, and Isabelle Thibau, MPH, discuss the National Eczema Association's EczemaWise app and how it can be used by both clinicians and patients.

The expanded voluntary recall is due to the potential microbial contamination of Burkholderia cepacia complex.

In part 3 of this Case-Based Roundtable supplement, Seemal Desai, MD, FAAD; Ted Lain, MD, MBA, FAAD; and Pearl Grimes, MD, FAAD, discuss real-world vitiligo scenarios, offering diagnostic insights, therapeutic strategies, and practical pearls from their own experiences.
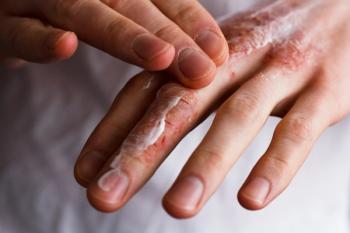
LEO Pharma's delgocitinib cream for chronic hand eczema is now available to prescribe in the US.

In part 2 of this Case-Based Roundtable supplement, Seemal Desai, MD, FAAD; Ted Lain, MD, MBA, FAAD; and Pearl Grimes, MD, FAAD, discuss real-world vitiligo scenarios, offering diagnostic insights, therapeutic strategies, and practical pearls from their own experiences.

In part 1 of this Case-Based Roundtable supplement, Seemal Desai, MD, FAAD; Ted Lain, MD, MBA, FAAD; and Pearl Grimes, MD, FAAD, discuss real-world vitiligo scenarios, offering diagnostic insights, therapeutic strategies, and practical pearls from their own experiences.

Vimal Prajapati, MD, FRCPC, DABD, highlighted key findings from the THRIVE-AA trials, noting the efficacy of deuruxolitinib 8 mg BID, its favorable safety profile, and its positioning alongside other JAK inhibitors.

Vimal Prajapati, MD, FRCPC, DABD, reviews the mechanism of action, clinical trial outcomes, monitoring considerations, and safety profile of recently available deuruxolitinib.

Renata Block, DMSc, MMS, PA-C, and Isabelle Thibau, MPH, of the National Eczema Association discuss how the EczemaWise app integrates patient-reported outcomes, validated tools, and real-world data to improve collaboration between patients and clinicians in managing eczema.

Members of Elevate-Derm Alliance and the SkinSync podcast discuss the inspiration behind the podcast and how it best serves dermatology clinicians.

Explore innovative biologic treatments for prurigo nodularis, enhancing patient quality of life and breaking the itch-scratch cycle effectively.

At a Dermatology Times Case-Based Roundtable event, Pearl Grimes, MD, led discussions on diverse vitiligo cases spanning adult, pediatric, and rapidly progressive disease, emphasizing individualized treatment strategies.

Robert Koppel, MD, led Case-Based Roundtable attendees through 3 complex cases of atopic dermatitis that centered around available non-steroidal topicals and biologics.

Unlock exclusive insights from Elevate-Derm Summer 2025 and get expert pearls, emerging data, and clinical strategies at your fingertips.

At Elevate-Derm Summer, Buchi Neita, MCMSc, PA-C, discussed enhancing dermatologic care for patients with skin of color by recognizing characteristics in common conditions and offered strategies to build rapport with patients.

At Elevate-Derm Summer, Shanna Miranti, MPAS, PA-C, highlighted practical strategies for isotretinoin prescribing, addressed the impact of iPLEDGE hurdles, and introduced the SkinSync podcast.

At Elevate-Derm Summer, Jim Treat, MD, outlined diagnostic limitations of imaging and the importance of recognizing capillary malformation patterns linked to other diseases.

Walter Liszewski, MD, highlights the clinical impact of patch testing and shares strategies for selecting and dosing JAK inhibitors at Elevate-Derm Summer.

At a recent Dermatology Times Case-Based Roundtable event, Stephanie Simmerman, DNP, APRN-C, discussed treatment selection, access challenges, and more for patients with vitiligo.

Experts share how this year's RAD Conference showcased cutting-edge advancements in atopic dermatitis to enhance patient care and treatment strategies.

At a recent Dermatology Times Case-Based Roundtable event, David Cotter, MD, PhD, reviewed treatment considerations for older patients with moderate to severe PsO who are candidates for biologic therapy.

Anabela Cardoso, MD, reflects on specific data from the phase 3b ADmirable trial evaluating the safety and efficacy of lebrikizumab in patients with moderate to severe AD and pigmented skin.

At a recent Dermatology Times Case-Based Roundtable event, Andrew Baker, PA-C, MBA, explored the management of skin cancers with GEP testing through 4 complex cases.

Renata Block, DMSc, MMS, PA-C, interviews Andrew Baker, PA-C, MBA, to discuss his role as a sub-investigator in Castle Biosciences’ UTILISE study for cSCC.