Delgocitinib Now Available in the US for CHE
Key Takeaways
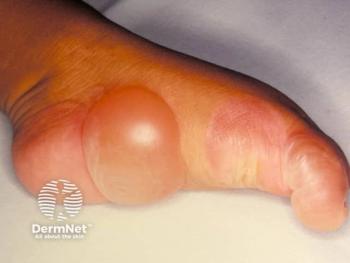
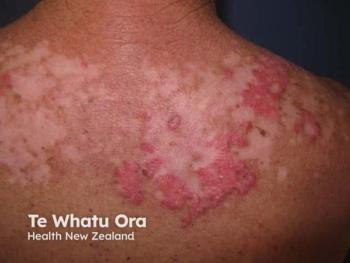
- Delgocitinib cream is the first FDA-approved treatment for moderate to severe chronic hand eczema in adults in the US.
- Phase 3 trials, DELTA 1 and DELTA 2, showed significant efficacy of delgocitinib, with higher success rates compared to vehicle cream.
LEO Pharma's delgocitinib cream for chronic hand eczema is now available to prescribe in the US.
The FDA approved delgocitinib on July 23, 2025, for patients with CHE who have not responded adequately to prior topical corticosteroids or for whom corticosteroid use is not advisable. The approval makes delgocitinib cream the first and only approved therapy in the US for CHE in adults.1
The approval of the steroid-free, topical pan-JAK inhibitor is supported by positive data from the phase 3 DELTA 1 (
The primary measure of success was achieving an Investigator’s Global Assessment for CHE Treatment Success (IGA-CHE TS)—a score of 0 (clear) or 1 (almost clear) with at least a 2-point improvement from baseline.
In DELTA 1, 20% of patients treated with delgocitinib reached this end point, compared to 10% in the vehicle group. In DELTA 2, 29% of delgocitinib-treated patients achieved success versus just 7% using vehicle cream. Both results were statistically significant, supporting the efficacy of delgocitinib in achieving visible skin improvement.
"It is truly exciting that topical delgocitinib is now FDA-approved in the United States for patients suffering from chronic hand eczema," said Christopher Bunick, MD, PhD, associate professor of dermatology at Yale School of Medicine in New Haven, Connecticut, and editor in chief of Dermatology Times, in a previous statement. "Delgocitinib represents the first approved medication specifically indicated for chronic hand eczema of all subtypes. This is a tremendous win for CHE patients who need a highly efficacious non-steroidal therapy they can use safely."
Additional data on delgocitinib from the DELTA 3 (
References
- Anzupgo (delgocitinib) cream is now the first and only FDA-approved treatment for moderate-to-severe chronic hand eczema (CHE) in adults. News release. LEO Pharma Inc. July 23, 2025. Accessed September 8, 2025.
https://www.businesswire.com/news/home/20250723115252/en/ANZUPGO-delgocitinib-Cream-Is-Now-the-First-and-Only-FDA-Approved-Treatment-for-Moderate-to-Severe-Chronic-Hand-Eczema-CHE-in-Adults - Bissonnette R, Warren RB, Pinter A, et al. Efficacy and safety of delgocitinib cream in adults with moderate to severe chronic hand eczema (DELTA 1 and DELTA 2): results from multicentre, randomised, controlled, double-blind, phase 3 trials. Lancet. 2024;404(10451):461-473.
doi:10.1016/S0140-6736(24)01027-4 - Gooderham M, Molin S, Bissonnette R, et al. Long-term safety and efficacy of delgocitinib cream for up to 52 weeks in adults with chronic hand eczema: results of the phase 3 open-label extension DELTA 3 trial following the DELTA 1 and 2 trials. J Am Acad Dermatol.
doi:10.1016/j.jaad.2025.03.008
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.