Drug Watch
Latest News
Latest Videos

CME Content
More News

A phase 2 trial reveals soficitinib's potential to significantly improve symptoms and quality of life in patients with moderate to severe AD.

The FDA’s decision was based on litifilimab’s phase 2 skin disease activity data.

Ritlecitinib shows promising facial repigmentation in vitiligo patients, with consistent efficacy across diverse demographics in a phase 2b study.
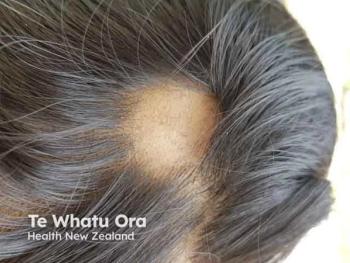
Aclaris Therapeutics' ATI-2138 shows promising rapid hair regrowth in severe alopecia areata models, highlighting its potential as a novel treatment option.

Amlitelimab shows promise as a long-term therapy for atopic dermatitis (AD), offering effective results and favorable safety in recent clinical trials.

Atopia Therapeutics advances ATP-R13 as a promising oral therapy for atopic dermatitis (AD), showing significant efficacy and enhanced patent protection.
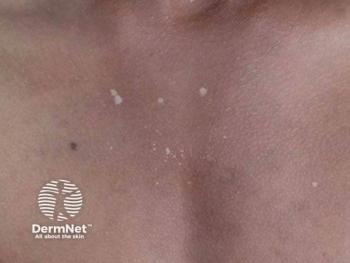
DELTA CARE 1 is the first phase 3 investigation of a pan–Janus kinase inhibitor specifically for lichen sclerosus.
BioMendics is advancing TolaSure in the TAMES-02 trial for EBS, aiming for rapid market access and potential expansion to other keratinopathies.
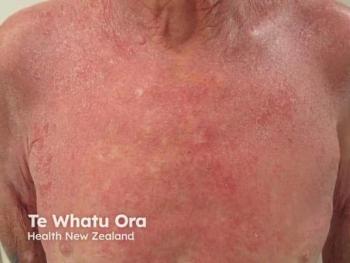
Quoin Pharmaceuticals seeks Breakthrough Medicine Designation for QRX003, aiming to provide the first treatment for Netherton Syndrome in Saudi Arabia.

Phase 1 cohort 4 data show soquelitinib demonstrated sustained clinical activity through 8 weeks, including in patients with prior systemic therapy exposure.
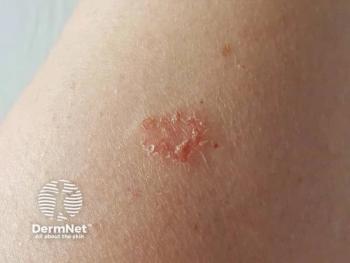
Almirall initiates a pivotal phase 3 trial for lebrikizumab, targeting nummular eczema and aiming to enhance treatment options for this challenging condition.

Jasper Therapeutics reveals promising briquilimab data, showcasing rapid disease control and safety in chronic spontaneous urticaria and chronic inducible urticaria.
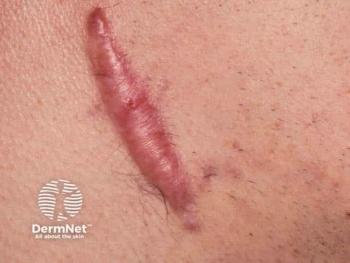
Mabwell’s investigational 9MW3811 is the first IL-11–targeting therapy to enter clinical evaluation for pathological scarring.

Explore the latest advancements in dermatology for 2026, featuring innovative therapies and treatments for skin conditions, hair loss, and more.
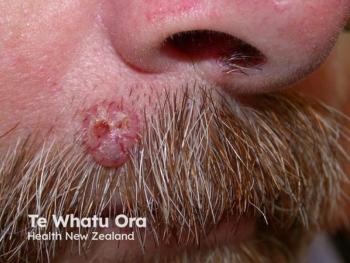
Topline results are expected in the first quarter of 2026.
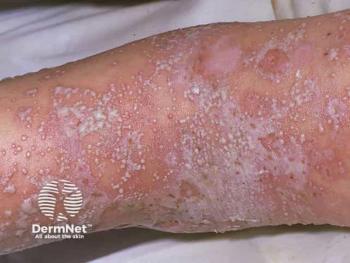
The BLA is supported by phase 3 data demonstrating rapid skin clearance and sustained disease control in patients with GPP.
Kymera Therapeutics reveals promising phase 1b results for KT-621, an oral STAT6 degrader, showing significant effects in moderate to severe atopic dermatitis.

The first treatment follows Zevaskyn’s FDA approval as the first autologous, gene-modified cellular sheet designed to address RDEB wounds.

Navigator Medicines advances NAV-240, a promising bispecific antibody for HS, targeting dual pathways for enhanced treatment efficacy.
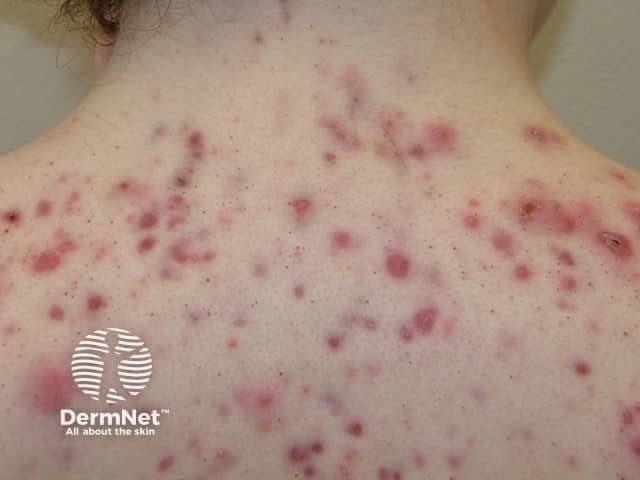
Citius Oncology’s IL-2 receptor–directed fusion protein offers rapid responses and pruritus improvement for relapsed or refractory stage I–III CTCL.

Lynk Pharmaceuticals reveals promising phase 2 trial results for LNK01004, a topical treatment showing efficacy and safety in moderate-to-severe atopic dermatitis.

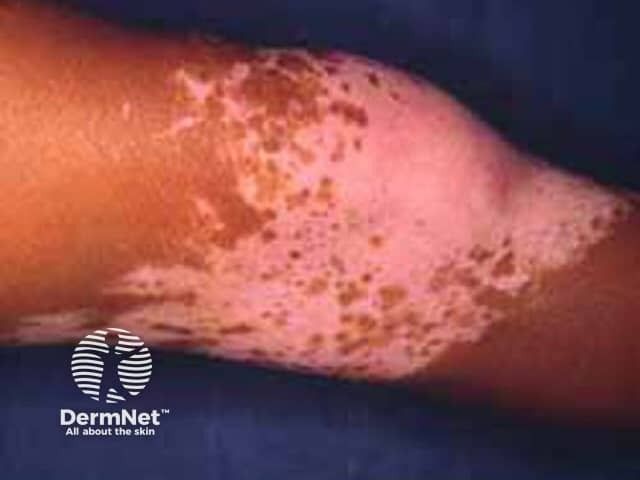
New research reveals ritlecitinib's potential to reverse scarring alopecias by targeting inflammation, offering hope for hair regrowth in autoimmune disorders.

Denifanstat emerges as a groundbreaking oral therapy for acne, targeting sebum production and inflammation, promising safer, effective treatment options.

Nemolizumab shows significant efficacy in reducing pruritus and improving skin clearance in patients with prurigo nodularis, offering new therapeutic hope.
The first-in-class mast cell–selective c-Kit inhibitor ALY-301 enters clinical testing for cold urticaria in Germany.