Drug Watch
Latest News
Latest Videos

CME Content
More News

Updated FDA policies eliminate many comparative efficacy trials, reducing biosimilar development time and cost by relying more on analytical testing.

In 2 replicate phase 3 trials, once-daily upadacitinib 15 mg significantly improved total and facial repigmentation in adults and adolescents with non-segmental vitiligo, with a safety profile consistent with prior indications.

Quoin Pharmaceuticals' QRX003 granted FDA Orphan Drug Designation, advancing treatment for Netherton Syndrome, a rare dermatological disease.

Azitra reveals promising preclinical results for ATR-01, a novel treatment targeting ichthyosis vulgaris, paving the way for future clinical trials.

The CHMP opinion follows recent US FDA approval, positioning Libtayo as the first immunotherapy approved for adjuvant treatment in high-risk CSCC.

Palvella surpassed enrollment goals in its Phase 3 SELVA trial, enrolling 51 patients at leading US vascular anomaly centers.
Lipella Pharmaceuticals' LP-10 shows promise as a targeted treatment for OLP, offering safety and efficacy in recent trials, according to Michael Chancellor, MD.

Sitryx Therapeutics advances SYX-5219 for moderate to severe AD, aiming for a first-in-class oral treatment with potential for lasting disease remission.

The approval represents the sixth FDA authorization for roflumilast since 2022.

Trial data show up to 30% of patients achieved complete response and 70% demonstrated some benefit from treatment.

Disc Medicine filed an NDA for bitopertin to treat erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLP) in patients ≥12 years.
The vaccine is designed to stimulate T cell responses against IDO1- and PD-L1–positive cells within the tumor microenvironment.
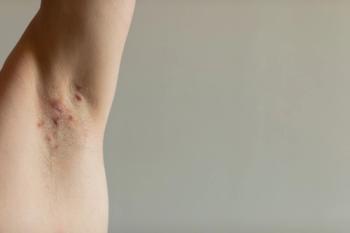
While VELA-1 achieved statistical significance under all analyses, VELA-2 narrowly missed its composite primary endpoint due to higher-than-expected placebo outcomes.

Fewer than 20% of eligible patients currently receive injectable therapies, highlighting the need for oral alternatives.

Phase 3 PROTOSTAR data support new indications, expanding treatment options for children impacted by chronic immune-mediated diseases.

Bambusa Therapeutics reveals promising phase I trial results for BBT001, targeting atopic dermatitis with dual-action bispecific antibodies.

TRIV-509 is a dual KLK5/7 inhibitor that aims to address barrier dysfunction, inflammation, and itch in moderate to severe AD.

Ruxolitinib cream has become the first topical JAK inhibitor available for pediatric atopic dermatitis (AD) in the US.
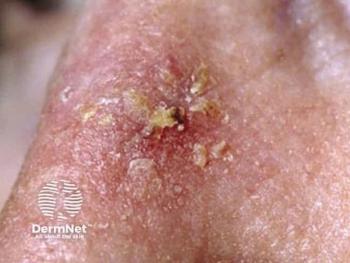
Biofrontera advances closer to expanding the Ameluz PDT label, as phase 3 follow-up data on actinic keratoses are expected next year.

The FDA's groundbreaking decision to waive clinical efficacy studies for a monoclonal antibody biosimilar revolutionizes drug approval, reducing costs and enhancing patient access.

Phase 3 trials demonstrated that icotrokinra met all primary and co-primary endpoints, including PASI 90 and IGA 0/1 responses.

The expanded voluntary recall is due to the potential microbial contamination of Burkholderia cepacia complex.

Amlitelimab shows promising phase 3 results in treating atopic dermatitis, achieving significant skin clearance and potential for infrequent dosing.
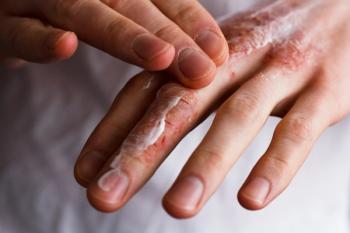
LEO Pharma's delgocitinib cream for chronic hand eczema is now available to prescribe in the US.

The FDA cited concerns about patient population heterogeneity and confirmatory trial design.