The United States Food and Drug Administration has granted Galderma’s nemolizumab a Breakthrough Therapy Designation following phase 2 results which suggests the drug is a considerable alleviant for pruritus associated with prurigo nodularis.

The United States Food and Drug Administration has granted Galderma’s nemolizumab a Breakthrough Therapy Designation following phase 2 results which suggests the drug is a considerable alleviant for pruritus associated with prurigo nodularis.

Significant variation exists in prescribing patterns among doctors managing atopic dermatitis, and the prescription of some drugs may go against dermatology guidelines, finds a recent study.

Patients with moderate-to-severe atopic dermatitis may have a 20% increased risk of atrial fibrillation, finds a recent study.
For patients with atopic dermatitis, access to dermatology care appears to be best in the West and poorest in the Midwest, shows a recent study looking at disparities in access to services across the United States.
Pain is a distinct and important symptom of atopic dermatitis experienced by almost two thirds of patients, reports a recent study.

Researchers recently analyzed insurance claims data to investigate the safety and efficacy of off-label immunomodulatory drugs for atopic dermatitis in real life practice. Read what they discovered here.

Atopic dermatitis presents differently depending on whether the patient is an adult or child. This means that different treatments may be more effective in an adult than children and vice versa.

A third of the most viewed eczema videos on YouTube give misleading and potentially harmful information, says a recent study.

Insights offer greater understanding around patient perspectives and desires that could prove useful to dermatologists in managing atopic dermatitis and psoriasis.

Researchers tested whether diluted bleach solution at the concentrations recommended for bleach baths is an effective antibacterial agent.
Clothing is a secondary skin and can influence the severity of skin conditions such as atopic dermatitis. But the antibacterial effectiveness of specialized clothing used to reduce disease symptoms varies considerably. Researchers recently decided to put these fabrics to the test - here's what they found.

As a class, JAK inhibitors look like the best oral targeted therapeutic option when topical cortical steroids and/or topical calcineurin inhibitors plus emollients fail.

Dermatologists need to consider modifying the appointment system to improve access to care for patients with atopic dermatitis flares, says this expert.
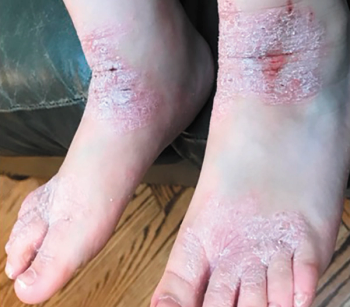
“Pretty much, I couldn’t go about my normal life,” says Benjamin Sun about a life-altering eczema flare up at age 9, which left him out of school and bedridden for weeks. Until now, children like Sun didn’t have an effective, safe option for long-term disease control. Read how dupilumab changed his quality of life, and what it can do for your pediatric patients with uncontrolled moderate to severe atopic dermatitis.

Patients with moderate-to-severe atopic dermatitis are at high risk of having inadequate disease control, according to a cross-sectional study in JAMA Dermatology.

A 13-year-old girl presented with a history of atopic dermatitis and alopecia totalis. Nine months after starting dupilumab, the patient regrew hair on 60 percent of her scalp.

Severe case of dupilumab-associated conjunctivitis successfully managed without discontinuing therapy.

In this article, Dr. Peter A. Lio discusses how recent advances in atopic dermatitis have led to a new standard of care, which aims to keep patients clear safely and maximize improvements in quality of life.

Adults with severe and mostly active atopic eczema are at a higher risk of developing cardiovascular disease, a population-based study shows.

Physicians bypass systemic treatments in moderate to severe cases of atopic dermatitis despite its potential to control symptoms.

Dupilumab-associated conjunctivitis may warrant discontinuing therapy for atopic dermatitis, a study shows.

Atopic dermatitis patients are likely more willing to adhere to treatment and achieve better outcomes when there is shared decision-making between the clinician and patient, according to an article in press in the Annals of Allergy, Asthma and Immunology.

In this slideshow, we highlight some of the most innovative and important clinical advances in the treatment of atopic dermatitis.

The biologic dupilumab is projected to be a cost-effective treatment for moderate-to-severe atopic dermatitis, shows a new study. (©Dzm1try/Shutterstock.com)

Topical vitamin A provides no benefit in treating atopic dermatitis, whereas topical vitamin D may actually exacerbate symptoms, according to an evidence-based review.