
Harold J. Brody, MD, provides his best tips and tricks for superficial and medium depth chemical peels in a presentation at the 2022 ASDS Annual Meeting.

Harold J. Brody, MD, provides his best tips and tricks for superficial and medium depth chemical peels in a presentation at the 2022 ASDS Annual Meeting.
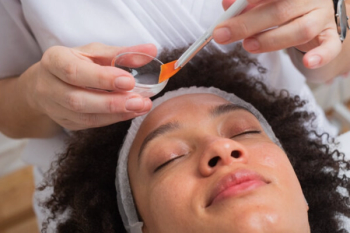
Pearl Grimes, MD, FAAD, dives deep into chemical peels, especially for your skin of color patients, at the 2022 ASDS Annual Meeting.

There are multiple factors predicting itch-specific quality of life in patients with chronic itch, according to a video oral abstract presentation at the 2022 ASDS Annual Meeting.

Authors of an oral abstract at the 2022 ASDS Annual Meeting presented their findings on the demographics of who requests total body skin exams to screen for skin cancer.

A 13-year-old boy presenting an asymptotic dark violaceous infiltrated nodular lesion located on the left cheek was diagnosed with blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy that often presents with skin manifestations.

Allergan Aesthetics announced the US Food and Drug Administration has approved the hyaluronic acid filler for jawline definition improvement.

Theodore Rosen, MD, details his top tips for treating hidradenitis suppurativa.

Theodore Rosen, MD, presents the best therapies for central centrifugal cicatricial alopecia and fontal fibrosing alopecia.

In this video interview, Jean Bolognia, MD, discusses her recent presentation at the 2022 Society of Dermatology Physician Assistants Annual Summer Meeting regarding how to identify the various cutaneous manifestations of lupus.

In part two of her presentation at the 2022 SDPA Annual Summer Meeting, Shanna M. Miranti, MPAS, PA-C, discusses the best treatment regimen for your rosacea patients.

Shanna M. Miranti, MPAS, PA-C, details how to determine which medications, products, and overall regimen will work for your acne patients.

Melissa Mauskar, MD, details how to handle difficult situations and conversations within your practice at the 2022 Society of Dermatology Physician Assistants Annual Summer Meeting in Austin, Texas.

Imagery is an essential part is diagnosing and documenting your patients’ skin. Joe Monroe, MPAS, PA, presented on how to effectively use your cell phone camera at the 2022 SDPA Annual Summer Meeting held June 16-19, 2022, in Austin, Texas.

The Society for Dermatology Physician Assistants (SDPA) begins its summer dermatology conference today, which will continue until Sunday, June 19, 2022, in Austin, Texas. Follow along for coverage.

This episode of The Cutaneous Connection podcast, sponsored by Incyte, explores the world of vitiligo. From symptoms to current treatment dissatisfaction and the latest research, Pearl Grimes, MD, FAAD, explains where we are now with this pigmentary disorder and where we are headed.

In this episode of DermView, Adelaide Hebert, MD, highlights both emollients and moisturizers. She also talks about bathing and how often a child should bathe if they have eczema.

Gary Linkov, MD, dives into the topic of hair loss.

In this month's OJM Group video series, Jason O'Dell, MS, CWM explains cash balance plans.

In this episode of DermView, Adelaide Hebert, MD, explains the clinical manifestations of eczema, how the chronic the condition is, and the benefits of using ceramide cream for management.

This episode of The Cutaneous Connection podcast, Gary Linkov, MD, dives into the topic of hair loss.

In this episode of DermView, Adelaide Hebert, MD, explains how eczema can lead to growth issues in pediatric patients, how environmental factors may play a role in flare ups, and more.

In this episode, Marc Serota, MD, discusses his research regarding type 2 inflammation, the future of treating it as 1 condition or 2, and more!

Coppertone has issued a voluntary recall for 12 lots of its aerosol sunscreen, citing detected levels of the carcinogen benzene.
Galderma and Sofregen Medical announced they have entered into an agreement to co-develop a novel line of silk-based biostimulators that will enable immediate volume restoration and provide structure for new tissue generation.

This week’s edition of The Mainstream Patient features stories about PFR fillers, blue LED light therapy, female hair loss, infected piercing care, plus more.

ICYMI, some of this week’s featured content includes a new podcast episode on molecular targeting of biologics, and articles on pipeline updates for melanoma, treating various skin conditions in skin of color patients, and more.

Catch up on all of the latest news coming out of the Skin of Color Update 2021.

This week’s edition of The Mainstream Patient features stories about Botox stigma, treating skin of color patients, laser hair removal, skin care microdosing, plus more.

This episode highlights findings from an investigative study exploring the molecular targeting of biologics for psoriasis, the IL-17 drug class, how bimekizumab is different, plus more.

Incyte announced the FDA has approved its topical selective Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib (Opzelura) for the treatment of mild to moderate atopic dermatitis.

Published: June 11th 2020 | Updated:

Published: October 21st 2021 | Updated:

Published: February 1st 2021 | Updated:

Published: June 17th 2022 | Updated:

Published: February 18th 2021 | Updated:

Published: January 25th 2021 | Updated: