
Menlo and Foamix announces shareholders have voted to approve the proposed merger between the two companies. Anticipated finalization of the merger is March 2020.

Menlo and Foamix announces shareholders have voted to approve the proposed merger between the two companies. Anticipated finalization of the merger is March 2020.

Sol-Gel announces updates to their drug pipeline and their goals for 2020, which include submitting multiple provisional patents and NDAs for the treatment of a variety of skin conditions.


The FDA announced it has accepted a priority review of dupilumab for the treatment of moderate-to-severe AD in pediatric patients 6 to 11 years of age. The drug is already approved for treatment of AD in adolescents and adults.

Medical device company Soliton presents results from their 12-week study looking at the treatment of fibrotic scars via their RAP device at Maui Derm.

Hovione recently published results of a phase 2b study showed a topical gel formulation of 1% and 3% minocycline proved to be an effective and safe treatment for papulopustular rosacea.

South Florida practitioner Dr. Susan Weinkle received the JDD Outstanding Educator & Mentor in Dermatology at the ODAC held Jan.17-20 in Orlando, Fla. The award recognizes continuing contributions to the dermatology industry through education.

Crown Laboratories has announced business division restructuring to establish themselves in the global skin care and dermatology market.

Medical device company, Soliton will present results from their 12-week study looking at the treatment of fibrotic scars using their RAP device at Maui Derm Jan. 25-29.
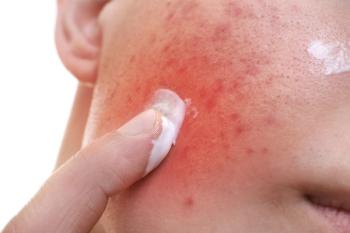
Recent phase 3 clinical trials reveal positive results for tazarotene lotion 0.045% in patients with moderate-to-severe acne.

Sol-Gel�’s new investigational microencapsulated benzoyl peroxide and tretinoin combination cream, Twyneo, for the treatment of acne vulgaris shows promising results from recently released phase 3 data with both clinical trials meeting all primary endpoints.

Trifarotene may become the European Union’s first new retinoid molecule acne treatment for the face and trunk in 25 years following upcoming individual member state marketing approvals in 2020.
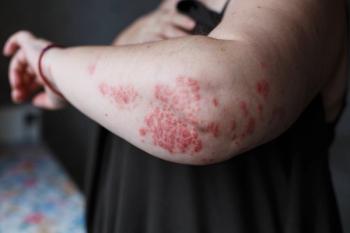
Results from an EU phase 3 clinical trial of a combination of betamethasone dipropionate and calcipotriene cream showed improved patient convenience, quality of life and efficacy compared to a betamethasone dipropionate and calcipotriene gel for patients with plaque psoriasis.
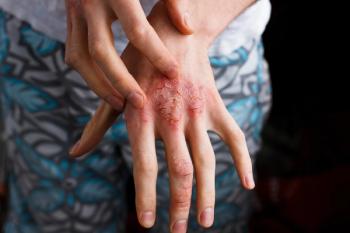
LEO Pharma has recently announced that all three phase 3 studies examining the safety and efficacy of the atopic dermatitis investigational drug tralokinumab met all of its primary and secondary endpoints, leading way to the company now seeking marketing authorization for the drug.
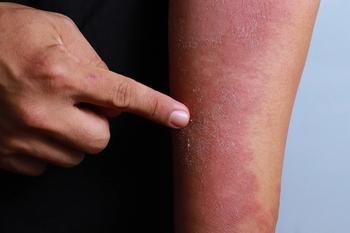
The U.S. FDA has granted Dermira with a Fast Track designation for its atopic dermatitis drug lebrikizumab following the start of phase 3 clinical trials to examine the efficacy, tolerability and safety of the drug.

A recent study examines the safety and efficacy of picosecond laser as a melasma treatment during a time when lasers are increasingly being used to treat the condition.

Newly published results from a phase 3 study demonstrate the superiority of bimekizumab over adalimumab for plaque psoriasis.
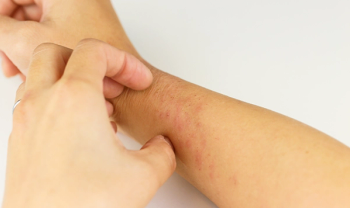
The United States Food and Drug Administration has granted Galderma’s nemolizumab a Breakthrough Therapy Designation following phase 2 results which suggests the drug is a considerable alleviant for pruritus associated with prurigo nodularis.
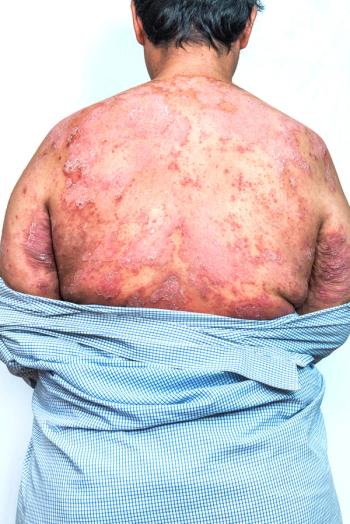
Leo Pharma A/S and Portal Instruments are now collaborating on a new needle-free handheld jet injector for patients that self-administer medicines for cutaneous diseases and ailments.

A recent study shows promising results for using ultrasound to examine hand tendon and joint inflammation in psoriatic arthritis patients.

A recent preclinical trial reveals that oxymetazoline in combination with pulsed dye laser may increase vascular shutdown in patients with rosacea and other cutaneous vascular diseases.