SanovaWorks announces they are moving their annual Skin of Color Update 2020 to a virtual setting amid various other live event cancellations due to the COVID-19 pandemic.

SanovaWorks announces they are moving their annual Skin of Color Update 2020 to a virtual setting amid various other live event cancellations due to the COVID-19 pandemic.

In this week's edition, we feature articles about expired sunscreen, uses for cortisone cream, Johnson & Johnson deciding to stop selling skin lightening products overseas, a warning from the FDA about possible toxic hand sanitizers, plus more.

The U.S. Food and Drug Administration has approved a single-dose 300 mg pre-filled pen version of dupilumab (Dupixent, Sanofi and Regeneron) for the treatment of atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis (CRSwNP) in U.S. patients 12 years and older.
Almirall announces the U.S. Food and Drug Administration has approved the updated label for their oral antibiotic sarecycline (Seysara) for treatment of acne vulgaris to help promote the appropriate use of the drug to aid in preventing antimicrobial resistance commonly warned when using antibiotics

ICYMI, this week’s edition features a video series on social media use in dermatology, the launch of our new podcast, as well as articles about FDA approvals for acne and cSCC, progress in epidermolysis bullosa, new rosacea management treatment, plus more.
The U.S. Food and Drug Administration has approved the Abbreviated New Drug Application for topical retinoid adapalene gel USP, 0.3% from Alembic Pharmaceuticals and its joint venture Aleor Dermaceuticals for treatment of acne vulgaris.
Tazarotene lotion 0.045% (Arazlo, Ortho Dermatologics) is officially launched in the U.S. following approval by the U.S. Food and Drug Administration in late 2019 for the treatment of acne vulgaris in patients 9 years and older.

The U.S. Food and Drug Administration has approved pembrolizumab for cutaneous squamous cell carcinoma whose disease has shown to not be curable by radiation or surgery.

In our first episode of The Cutaneous Connection, Adam Friedman, M.D., FAAD, discusses his recent presentation at the first virtual American Academy of Dermatology meeting about cannabinoids use in dermatology.

In this week's edition, we feature stories about dry brushing, the difference between psoriasis and eczema, comedone extractors, at-home microneedling, plus more.

ICYMI, this week’s edition features articles about laser therapy for BCC, the growing interest in JAK-STAT inhibition, tips on how to sell your practice, apremilast for scalp pruritus, plus more.

Evidence Skincare is now offering consumers a complementary skin survey to help identify consumers' problem areas, recommend skincare regimens and provide suggestions on what products might work best for them.

The Inaugural Pediatric Dermatology 2020: Best Practices and Innovations Conference is now open for registration and scheduled to take place June 27th, offering seven free CME credits.

A startup from Purdue University seeks to make handwashing easier and more accessible during COVID-19 through its new portable bedside sink technology developed by Project Process.

In this week’s edition, we feature stories about black-owned skincare lines, polyglutamic acid, hair antioxidants, a skincare company making affordable custom topical prescriptions, plus many more.

ICYMI, we've curated a list of all the stories we've published throughout the past week. Check out this article for more Dermatology Times content.

LEO Pharma will soon begin marketing its monoclonal antibody tralokinumab for atopic dermatitis with the announcement that the EMA has accepted the marketing authorization application for the drug.
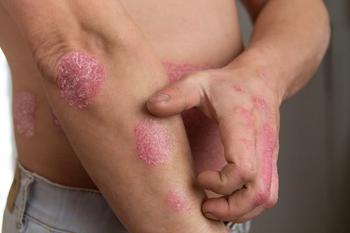
Dermavant announces the enrollment completion for their long-term safety study investigating tapinarof in adults with plaque psoriasis as a part of the PSOARING 1 and PSOARING 2 phase 3 program.

AAD announces two physicians are recipients of the "Patient Care Hero" award for their work in implementing a teledermatology program in the U.S. Army program two decades ago.

ForBabs introduces their X-Static hairbrush that keeps fly-aways and frizz at bay.

In this week’s edition, we highlight stories about ways to support people of color in the beauty industry aside from purchasing products, the safety of LED light therapy, dermaplaning at home, why your skin might be acting up right now, plus more.

In case you missed it, this week we featured stories involving blastic plasmacytoid dendritic cell neoplasm (BPDCN), development of a biosimilar to BOTOX, a new nonmelanoma treatment device, the latest phase 3 results for abrocitinib in atopic dermatitis, results from a long-term dabrafenib and trametinib study in stage 3 BRAF-mutated melanoma, plus many more.
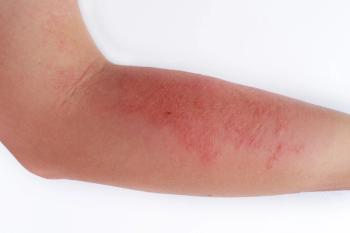
Full results from the second of two trials investigating the JAK1 inhibitor abrocitinib as a monotherapy for treatment of moderate-to-severe atopic dermatitis demonstrate statistical superiority of both doses compared to placebo.
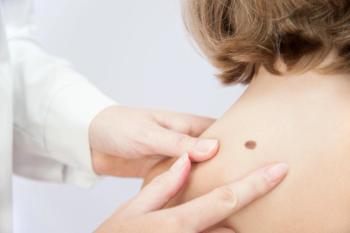
Results presented at the recent ASCO 2020 Virtual Scientific Program demonstrate the long-term relapse-free and survival benefit of dabrafenib (Tafinlar, Novartis) and trametinib (Mekinist, Novartis) as a combination treatment following surgery in stage III BRAF V600E/K-mutant melanoma patients.
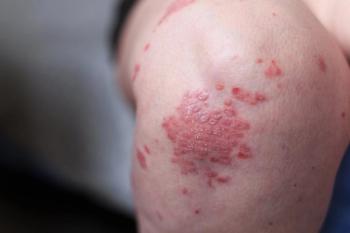
AbbVie announces they have filed applications with the U.S. FDA and EMA for the approval of the JAK inhibitor upadacitinib for the treatment of adult active psoriatic arthritis.

Mylan has agreed to move forward with Revance in the development plan of a biosimilar to onabotulinumtoxinA (BOTOX, AbbVie) under the 351(k) regulatory pathway from the U.S. FDA.

In honor of June being Acne Awareness Month, Thayers has launched a new line of acne-tailored skincare products.

The American Society for Laser Medicine and Surgery announces a new president and officers will be joining their Board of Directors for 2020-21.

This week's edition features articles about pore strips, Zoom skin cancer checks, differences between mineral and chemical sunscreens, acupuncture for acne and many more.

To keep you up to date with the industry’s latest, we’ve gathered together stories from this week involving glycolic-salicylic acid serum, pediatric multisystem inflammatory syndrome, FDA approvals for dupilumab and minocycline, the latest study results for tapinarof, plus more.