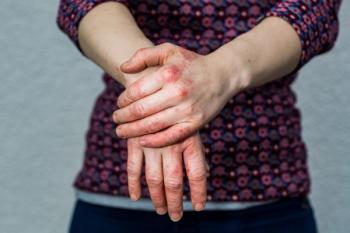
The U.S. Food and Drug Administration (FDA) has granted JAK-inhibitor delgocitinib cream (LEO Pharma) Fast Track Designation for chronic hand eczema.

The U.S. Food and Drug Administration (FDA) has granted JAK-inhibitor delgocitinib cream (LEO Pharma) Fast Track Designation for chronic hand eczema.

A recently published study finds from 2008 to 2017, primary care physicians received only a slightly greater rise in compensation than specialists under the 2010 Affordable Care Act.

SilcSkin introduces their Multi-Area Pads to help treat fine lines and wrinkles in the mouth area.

The European Commission has approved secukinumab (Cosentyx, Novartis) for treatment of moderate-to-severe plaque psoriasis in pediatric patients 6<18 years in the European Union.

Demandforce introduces their mobile app, available free to all Demandforce users, aimed at making practice operations and the lives of physicians easier through streamlined communication and office efficiency.

This week’s edition of The Mainstream Patient features stories about sun spots, niacinamide, proper body hair removal, psoriasis-related mental health treatment, plus more.

ICYMI, some of the content featured this week includes results from trials investigating topical minocycline and calcipotriene betamethasone foam. Also, FDA approval of a combination treatment for advanced melanoma, a new picosecond laser, plus more.

The U.S. Food and Drug Administration has approved atezolizumab plus cobimetinib and vemurafenib for treatment of BRAF V600 mutation-positive advanced melanoma. Studies show the combination treatment prolonged patients’ lives by 15 months without disease worsening.

The new 730-nm picosecond laser demonstrates strong efficacy and safety in darker skin types for treatment of endogenous pigmentary disorders, according to a recent case study.

The FDA expands their list of hand sanitizers found to contain methanol, a toxic chemical, which, when absorbed through the skin or ingested, can lead to adverse events.

Cath O'Niell, chief scientific officer at SkinBioTherapeutics and professor of translational dermatology at the University of Manchester, discusses her recent research on developing probiotics to treat psoriasis and other inflammatory skin diseases.
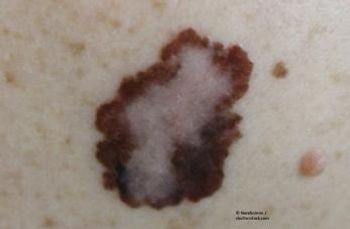
A recently published pooled 10-year analysis shows pembrolizumab demonstrates long-term survival benefits for advanced melanoma patients regardless of BRAF V600E/K mutation status or history of BRAFi, with or without MEKi therapy.

This week’s edition of The Mainstream Patient features stories about inflammation-linked aging, how to get rid of varicose veins, Kybella for under the chin, plus more.

ICYMI, some of the content featured this week includes a new episode of The Cutaneous Connection, how to eat for healthy skin, botulinum toxin as a potential treatment for scars, fat grafting, plus more.

The U.S. Food and Drug Administration has approved Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%, MC2 Therapeutics) for adult with plaque psoriasis. Wynzora is equipped with the MC2 Therapeutics’ patented PAD Technology and is currently under review in the EU.

This episode discusses a recent survey conducted by the International Society of Dermatology’s Climate Change Committee involving climate change and its effect on skin diseases throughout the world with one of its committee members, Sarah Coates, M.D.

DMK Skin Care launches new products at their U.S. DMK clinics to help combat the effects of summer on the skin.

This week’s edition of The Mainstream Patient features stories about the difference between physical and chemical sunscreen, everything you need to know about exfoliants, a complete guide to laser hair removal, plus more.

ICYMI, some of the stories featured this week include a look at the impact of COVID-19 in patients on biologics, a novel filler injection technique, FDA approvals for psoriatic arthritis, a potential biomarker of psychiatric adverse events with isotretinoin use, plus more.

The Society of Pediatric Dermatology introduces a new president and executive committee for 2020-2021 during their virtual 45th Annual Meeting.

Guselkumab becomes the first IL-23 inhibitor approved for treatment of psoriatic arthritis by the U.S. Food and Drug Administration (FDA) following positive phase 3 results from two trials investigating the safety and efficacy of the drug.

The U.S. Food and Drug Administration has approved Mylan and Fujifilm Kyowa Kirin Biologics’ adalimumab-fkjp as a biosimilar to AbbVie’s adalimumab for a variety of inflammatory disease including plaque psoriasis and psoriatic arthritis.

This week's edition features stories in consumer media involving sunscreen terms, facial erythema, inner thigh chafing, pityrosporum folliculitis, how to treat "maskne," plus more.

ICYMI, some of the stories featured this week include a look at the impact of apremilast treatment for psoriasis on a patient who also tested positive for COVID-19, exploring IL-17 inhibition for rosacea, FDA approvals for a cellulite injectable and atopic dermatitis treatment, plus more.
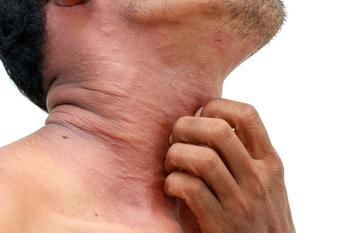
The U.S. Food and Drug administration has accepted the Biologics License Application for tralokinumab (LEO Pharma) for moderate-to-severe atopic dermatitis and is expected to make a decision in the second quarter of 2021.

With summer in full swing, this episode takes a look at how social media can influence sun protection use in teens and young adults, and why certain ingredients in sunscreens are considered controversial.

The U.S. FDA has approved collagenase clostridium histolyticum-aaes (Qwo, Endo International), making it the first FDA-approved injectable treatment for moderate-to-severe cellulite in the buttocks in adult women.

This week we feature articles about the rise in plastic surgery procedures and 'tweakments' during the COVID-19 pandemic, uses for aloe vera in skincare, retinol use for acne, plus more.

ICYMI, this week’s edition features a warning from the FDA about potentially toxic hand sanitizers, the latest data from the phase 3 bimekizumab trial for psoriasis, gene expression profiling for skin cancer, PRP for periorbital rejuvenation, plus more.

The U.S. Food and Drug Administration (FDA) has issued a warning about potentially toxic hand sanitizers.