Drug Watch
Latest News
Latest Videos

CME Content
More News
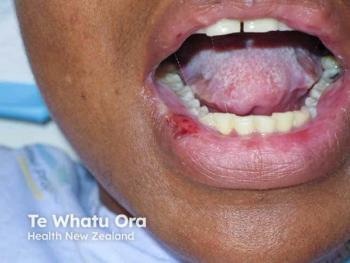
The open-label trial showed dusquetide produced similar results to apremilast in reducing oral ulcers, pain, and ulcer duration.

PC111, a novel therapy from Scinai, targets pemphigus vulgaris and SJS/TEN, offering rapid relief without immunosuppression.

VYNE Therapeutics reveals disappointing phase 2b trial results for repibresib gel in nonsegmental vitiligo, missing key efficacy endpoints.

Nektar Therapeutics advances alopecia areata treatment with FDA's Fast Track designation for rezpegaldesleukin, targeting immune modulation in chronic hair loss.

The approval makes delgocitinib cream the first and only approved therapy in the US for CHE in adults.

Replimune faces FDA challenges for RP1's approval in advanced melanoma, highlighting complexities in cancer drug development and regulatory scrutiny.

Johnson & Johnson submitted an NDA for icotrokinra, a promising new treatment for moderate to severe plaque psoriasis in adults and adolescents.

Exclusive Report: A comprehensive look at the latest FDA approvals and pipeline developments in dermatology from the first half of 2025.

FDA enhances transparency by publishing over 200 Complete Response Letters, offering insights into drug approval processes and common deficiencies.

Jasper Therapeutics reveals promising results from briquilimab trials for chronic spontaneous urticaria, showcasing strong efficacy and safety despite manufacturing challenges.

VYNE Therapeutics updates on VYN202, revealing promising early results in psoriasis treatment despite a clinical hold due to safety concerns.

A new paper highlights Alphyn Biologics' Zabalafin Hydrogel as a promising multitarget therapy for atopic dermatitis, addressing inflammation, itch, and bacterial complications.

Quoin Pharmaceuticals advances QRX003 for Netherton Syndrome, receiving FDA's RPD Designation and showing promising clinical results.

Nektar Therapeutics reveals positive results from the REZOLVE-AD study, showcasing rezpegaldesleukin's efficacy in treating moderate to severe AD.

Galderma launches clinical trials for nemolizumab, targeting systemic sclerosis and chronic pruritus of unknown origin, addressing critical patient needs.

The new PDUFA date is set for September 2025.

Dupilumab gains FDA approval as the first targeted therapy for bullous pemphigoid, offering hope for effective treatment and improved patient outcomes.

Pediatric dermatologist Mercedes E. Gonzalez, MD, discusses roflumilast's promise as a safe, nonsteroidal treatment for atopic dermatitis in infants.
Mercedes Gonzalez, MD, discusses the promising INTEGUMENT-INFANT trial, exploring a new non-steroidal treatment for infants with atopic dermatitis.
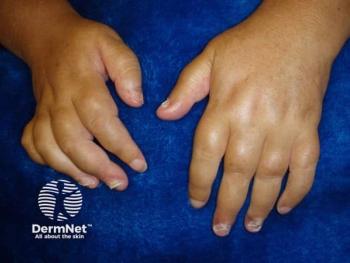
At the 2025 EULAR meeting, UCB presented new 3-year data on bimekizumab for PsA.
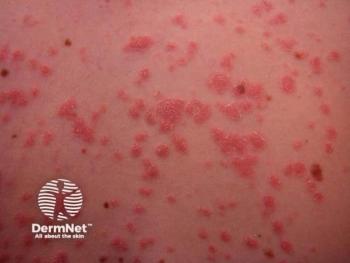
Zasocitinib emerges as a promising oral therapy for psoriasis and psoriatic arthritis, showcasing superior selectivity and safety compared to traditional JAK inhibitors.
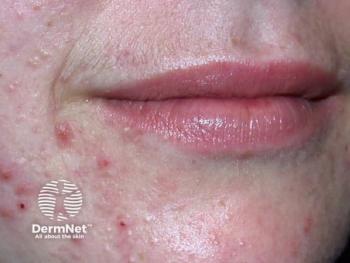
Sagimet Biosciences reveals denifanstat's promising phase 3 results, offering a novel oral treatment for moderate to severe acne with significant efficacy.

FDA approves Arcutis' roflumilast foam, a groundbreaking treatment for plaque psoriasis, offering a steroid-free, effective solution for patients aged 12 and older.

Quoin Pharmaceuticals advances QRX003 for Netherton Syndrome, receiving FDA clearance for a pivotal trial to assess its effectiveness and safety.

Accropeutics reveals promising Phase 2 trial results for AC-201, a selective TYK2/JAK1 inhibitor, showing significant efficacy in treating plaque psoriasis.