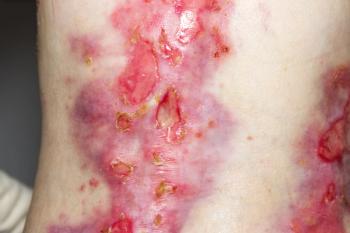
Chiesi Global Rare Diseases recently announced the approval of the topical treatment.

Chiesi Global Rare Diseases recently announced the approval of the topical treatment.

Here is a recap of the drugs and treatments approved to keep in mind for the new year.

Roflumilast foam 0.3% is the first FDA-approved drug for seborrheic dermatitis with a new mechanism of action in over 2 decades. Christopher Bunick, MD, PhD; Shawn Kwatra, MD; and Peter Lio, MD, share expert insights.

The confirmatory phase 3 trial demonstrated significant efficacy of TO-208 versus a placebo.

Adbry (tralokinumab-LDRM) is now the first and only FDA-approved biologic for AD binding to and inhibiting IL-13.

Biolojic’s BD9 is a dual specific antibody that can block both Thymic stromal lymphopoietin and IL-13.

The study evaluated the efficacy of AIV001 in superficial, nodular, and mixed BCC tumors.

One of the specific areas of focus will be non-melanoma skin cancer.

In January 2023, adalimumab-atto became the first biosimilar product available for the reference product adalimumab.

KT-474 is a first-in-class, investigational IRAK4 degrader.

The US license period will start on February 22, 2025.

The sNDA is supported by positive data from the phase 3 INTEGUMENT-1 and INTEGUMENT-2 trials.

The FDA’s PDUFA target date is May 25, 2024.

One notable change: CVS Caremark has removed the Humira biosimilar Amjevita and now prefers Hyrimoz and an unbranded biosimilar.

Jared Gollob, MD, Chief Medical Officer of Kymera Therapeutics, spoke with Dermatology Times to discuss these trial results.

There are currently no FDA-approved therapies for the rare, genetic disease.

Shawn Kwatra, MD, shares his thoughts on the positive phase 3 data of OLYMPIA 2.

Data from Novartis will be presented at the 2023 American College of Allergy, Asthma, and Immunology Scientific Meeting.

HADLIMA is currently indicated for psoriatic arthritis, plaque psoriasis, and hidradenitis suppurativa, among others.

The approved indication makes Wezlana the first approved interchangeable biosimilar to Stelara.

The US Food and Drug Administration has approved IDP-126 gel for patients with acne.
The CRL states that additional efficacy data is needed in order to support approval.

Bimekizumab is now the first and only approved IL-17A and IL-17F inhibitor for this indication.

The advancement is based on previous observations of preliminary clinical efficacy. There are currently no FDA-approved therapies for this patient population.

Approval is based on data from Bristol Myers Squibb’s CheckMate – 76K trial.