- Dermatology Times, November 2025 (Vol. 46. No. 11)
- Volume 46
- Issue 11
Beyond Antibiotics: How Oral Denifanstat Could Reshape the Acne Pipeline
Key Takeaways
- Denifanstat targets fatty acid synthase, reducing sebum production and inflammation, offering a novel mechanism for acne treatment.
- Phase 3 trials showed significant efficacy, with a 60% reduction in total lesions and minimal adverse effects compared to placebo.
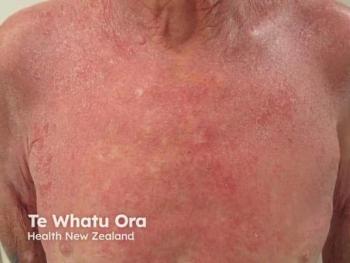
Denifanstat emerges as a groundbreaking oral therapy for acne, targeting sebum production and inflammation, promising safer, effective treatment options.
A potential game-changer in acne treatment is on the horizon. Denifanstat (ASC40), a first-in-class oral therapy developed by Ascletis Pharma Inc, is redefining how moderate to severe acne vulgaris could be managed, moving beyond the long-standing reliance on antimicrobials, hormones, and retinoids. By targeting a completely new biological pathway, this breakthrough therapy could usher in a new era of acne care. Phase 3 safety and efficacy results were unveiled at the
“Anytime there is a novel molecule or mechanism under development for a dermatology indication, we should all be excited for the potential therapeutic benefits.... It’s a few years away, but our patients have something coming down the road,” Neal Bhatia, MD, medical director of Therapeutics Clinical Research in San Diego, California, and former vice president of the American Academy of Dermatology, told Dermatology Times.
First-in-Class FASN Inhibitor Mechanism
Denifanstat is a first-in-class inhibitor of fatty acid synthase (FASN), a key enzyme in the de novolipogenesis pathway responsible for roughly 80% of the lipids that make up sebum. Bhatia compared this mechanism of action to “removing the fuel from the car so it cannot run.”
“It is well understood that sebum quantity and composition are key drivers of acne severity, and denifanstat has a direct impact on both by reducing and regulating the production of sebum,” he said. “This is different than topical clascoterone, which is an androgen receptor inhibitor intending to reduce sebum production, or spironolactone, which can directly impact androgens and receptors.”
Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Brooklyn, New York, and clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical Center, further explained the drug’s twofold mechanism of action, as it also suppresses the inflammation resulting from decreased cytokine secretion and Th17 differentiation. This is similar to retinoids, without the associated dryness or epidermal turnover, according to Bhatia.
“Denifanstat represents a novel mechanism of action, since every patient with acne has an elevated sebum level, with limited options that effectively address this, and it represents a significant treatment that could make a difference in all acne patients,” Bhatia said.
Denifanstat’s Phase 3 Data at a Glance
The phase 3 trial (NCT06192264) was a randomized, double-blind, placebo-controlled, multicenter study conducted in China. A total of 480 patients with moderate to severe acne were randomly assigned 1:1 to receive either 50 mg of denifanstat orally once daily or placebo for 12 weeks, followed by a 40-week open-label safety study. Approximately 85.8% of the group began the trial with grade 3 disease (moderate), while 14.2% started with grade 4 (severe).
The study’s primary end points included Investigator’s Global Assessment (IGA) success (IGA score of 0 or 1 and ≥ 2-point reduction from baseline), the percent reduction in total lesion count, and the percent reduction in inflammatory lesion count at week 12. Key secondary end points included the percent reduction in noninflammatory lesion count.
Key Efficacy and Success Rates
Denifanstat met all key primary and secondary end points, with the results demonstrating both statistical and clinical significance compared with placebo. Notably, treatment effects were evident as early as week 4. At week 12, the denifanstatpatient group saw higher levels of IGA treatment success compared with placebo (33.17% vs 14.58%; P < .0001). There was also a nearly 60% reduction in total lesions, along with statistically significant decreases in inflammatory and noninflammatory lesion counts.
Reviewing the Clinical Safety Advantage
Denifanstat also demonstrated a favorable safety and tolerability profile across the entire trial. The incidence of treatment-emergent adverse events was comparable between the treatment and placebo groups (58.6% vs 56.3%). Most adverse effects were mild (grade 1) or moderate (grade 2), and no treatment-related or grade 3/4 drug-related serious adverse events were observed in the trial and safety follow-up. The most commonly reported ones, as noted by Baldwin, included dry skin (6.3% denifanstat vs 2.9% placebo) and xerophthalmia (5.9% denifanstat vs 3.8% placebo).
“More impressive than the efficacy is the safety, which will be a welcome addition to the oral therapeutic ladder for treating acne and hopefully reduce reliance on antibiotics and even hormonal therapies,” Bhatia added.
A Potential Replacement for Oral Antibiotics
“Sadly, the pipeline for acne treatments is drying up, and overall acne has been an underdeveloped disease market over the past 40 years,” Bhatia said. “For the last 10 years, dermatologists have been trying to reduce the reliance on oral antibiotics for controlling moderate to severe acne, and denifanstat seems to be a potential replacement for the over 5 million prescriptions written by dermatologists. Although the mechanisms are not the same, having another treatment option is very welcome.”
Sonia Batra, MD, clinical assistant professor at USC Keck School of Medicine, agreed and shared her excitement for a new tool in the arsenal that safely and effectively inhibits FASN, potentially becoming an alternative to antibiotics.
“Since the trial looked at oral denifanstat as a monotherapy, I am hopeful that combining it with existing treatment modalities may lead to even more impressive results,” she said.
Ascletis is currently completing pre–new drug application (NDA) consultations with the China National Medical Products Administration in hopes of submitting an NDA soon. The next phases will involve clinical trial sites in the US, making this first-in-class agent targeting lipid metabolism a potentially valuable option in the acne treatment landscape.
“Excess sebum production and inflammation are both foundational aspects of acne pathophysiology,” Baldwin added. “So we look forward to having this new agent in our acne toolbox.”
Reference
Xiang L. First-in-class FASN inhibitor denifanstat achieved all endpoints in the treatment of acne vulgaris: results from a phase III randomised placebo controlled trial. Presented at: European Academy of Dermatology and Venereology Congress 2025; September 17-20, 2025; Paris, France.
Articles in this issue
about 2 months ago
Dermatology Times November 2025 Print Recapabout 2 months ago
Q4 Investment Planning: What to Do Now to Reduce What You Owe in 20262 months ago
Skin Aging and Cellular Senescence2 months ago
Clinicians Debate True Sensitive Skin Care3 months ago
ORKA-001 Advances Toward Yearly Dosing3 months ago
Treating the Pain of Atopic DermatitisNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.