- Dermatology Times, November 2025 (Vol. 46. No. 11)
- Volume 46
- Issue 11
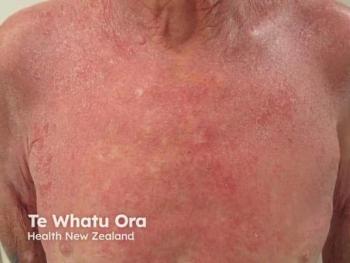
Psoriasis Care Evolves as Oral IL-23 Blockers Show Lasting Control
Key Takeaways
- Icotrokinra, an oral IL-23 receptor blocker, offers a significant advancement in psoriasis care, providing a needle-free option for patients.
- Defining remission and balancing efficacy with long-term safety are critical considerations in the evolving landscape of psoriasis treatment.
Experts discuss icotrokinra's potential to revolutionize psoriasis treatment with oral therapies, emphasizing efficacy, safety, and patient-centered care at EADV 2025.
At the
A New Mechanistic Frontier
For Adam Friedman, MD, professor and chair of dermatology at George Washington University in Washington, DC, icotrokinra’s distinction lies not just in its form factor, but its target. “One of the hot topics is going to be the first-ever oral peptide–based biologic that targets not IL-23, but the IL-23 receptor,” he said. “What I’m really excited about is that this gives our patients even more options beyond the plethora—or giant buffet—of biologic options, especially for those needle-phobic patients.”
That mechanistic nuance of receptor vs cytokine blockade could yield not only convenience but insight. As Friedman put it, “If you block the receptor, you are probably going to have a greater impact. Because even if you’re churning out a whole bunch of signals, if it can’t get to its target, it can’t have a downstream influence.”
The implications stretch beyond psoriasis. Oral peptide–based biologics could represent a prototype for a new generation of immunomodulatory agents, potentially reshaping therapeutic expectations for inflammatory dermatoses from hidradenitis suppurativa to lichen planus.
Redefining Remission
Leon Kircik, MD, clinical professor of dermatology at Mount Sinai in New York, New York, underscored the importance and complexity of defining success. “We should be aiming at 100% clear—PASI [Psoriasis Area and Severity Index] 100. That’s our goal,” he emphasized. Yet, he added, “First, we need to define what remission is. Is remission on drug or off drug? How long is it? For the rest of time or for a period of time?”
Such questions remain central as oral IL-23 blockers demonstrate durable control beyond one year, as seen in recent long-term extension data. Kircik also highlighted the balance between efficacy and lifetime safety. “Psoriasis is a chronic disease. People are using the drugs long term or for their lifetime,” he said. “Certainly, we never know exactly what’s going to happen in 10 years or 20 years.”
His perspective mirrors a growing consensus that remission is not binary but dynamic, evolving with patient preference, disease chronicity, and ongoing safety vigilance.
Safety Beyond the Clinical Trial
From Houston, Texas, clinical researcher Harrison Nguyen, MD, MBA, MPH, reflected on how tightly controlled trial data translate, or sometimes don’t, into everyday practice. “How we interpret the safety data in a trial usually does translate to the real world,” he said, “but there are some nuances because when we do a clinical trial, it is a very controlled setting.”
Still, Nguyen found reassurance in the early safety signals. “Whenever we see safety profiles or adverse events comparable in the treatment arm to placebo—that’s incredible. That really signals a very favorable safety profile and gives us reassurance, especially for a new treatment.”
He also noted that withdrawal and recapture data—the ability to regain efficacy after a dosing gap—are “critical because they’re reflective of the real world.” Such insights are invaluable in the context of insurance interruptions, patient adherence, and complex comorbidities that characterize real-world psoriasis care.
The Pediatric and Psychosocial Lens
Mona Shahriari, MD, associate clinical professor and investigator at Yale School of Medicine in New Haven, Connecticut, reminded colleagues that psoriasis treatment is not merely about surface clearance. “There are risks to not treating the disease,” she said. “Having psoriasis early on may prevent you from interacting with your group of friends—these are tiny impacts, but over the course of someone’s life, it can actually change their entire life.”
Her comments highlight the psychosocial burden of psoriasis, especially in adolescents. For younger patients, oral agents offer not only efficacy but dignity—reducing stigma associated with injections or visible lesions. Shahriari also stressed long-term tolerability. “What do I not see? I’m not seeing a signal for diarrhea, nausea, acne, folliculitis, mouth ulcers, things that could be very problematic, especially in an adolescent population.”
For early-career clinicians, her insight is a reminder that safety extends beyond laboratory values; it includes quality of life, convenience, and developmental context.
Evidence Over Insurance
Finally, Aaron Farberg, MD, chief medical officer of Bare Dermatology in Dallas, Texas, brought the discussion back to first principles of medicine. “We’re not practicing insurance-based medicine. We should be practicing evidence-based medicine.”
Farberg’s confidence in emerging oral agents reflects a seasoned pragmatism. “Now, I can truly have that discussion with the patient: ‘We have a pill. We have an injection. Which one do you think is going to be best for you?’” Still, he remains cautious. “When I was young, of course you want to move forward and try the newest, latest, and greatest. Now, I’m a bit more cautious, and I want to see and review all that phase 3 data prior to utilizing a new therapy.”
His perspective resonates across the experience spectrum. For those just entering practice, it is a call to diligence. For veterans, it reaffirms the enduring role of clinical judgment amid rapidly evolving therapeutics.
Guardrails and the Road Ahead
Across the interviews, one theme was clear: Innovation demands infrastructure. Farberg, who also serves on the American Academy of Dermatology’s guideline committee, described their mission as “putting the guardrails up, but allowing all of these new medications to be utilized by us, the clinicians.”
As oral IL-23 blockers demonstrate durable efficacy and tolerability, new questions arise about sequencing, adherence, and combination strategies. For Kircik, real-world complexity is inevitable. “In real life, especially in dermatology, we always use drugs in combination.” Yet, he notes, “As long as the agency requires that we do the trials as monotherapy, it will never be like real life.”
Still, optimism among is high. Friedman sees the dawn of oral biologics as both a scientific milestone and a clinical liberation. “Every time we get a new drug, we learn a lot about a disease.”
The challenge and opportunity for clinicians lies not only in mastering mechanisms, but in translating them into meaningful patient outcomes.
Articles in this issue
about 2 months ago
Dermatology Times November 2025 Print Recapabout 2 months ago
Q4 Investment Planning: What to Do Now to Reduce What You Owe in 20262 months ago
Skin Aging and Cellular Senescence2 months ago
Clinicians Debate True Sensitive Skin Care3 months ago
ORKA-001 Advances Toward Yearly Dosing3 months ago
Treating the Pain of Atopic DermatitisNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.