Outlining the Clinical Development Strategy of TolaSure for EB Simplex
Key Takeaways
- TAMES-02 trial evaluates TolaSure for EBS, focusing on reducing blister burden, with a randomized, placebo-controlled design and crossover feature.
- BioMendics aims for breakthrough therapy designation and plans to expand TolaSure's application to other keratinopathies, leveraging its autophagy-mediated mechanism.
BioMendics is advancing TolaSure in the TAMES-02 trial for EBS, aiming for rapid market access and potential expansion to other keratinopathies.
“That pivotal trial is really ultimately our goal...We're really trying to get this on the market as quickly as we can for patients,” Karen McGuire, PhD, CEO and co-founder of BioMendics, said in
McGuire outlined the design and objectives of the ongoing
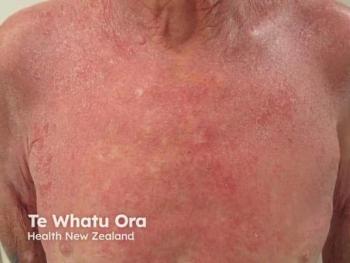
Unlike its predecessor, which relied on internal controls, TAMES-02 enrolls patients into 2 parallel groups receiving either active treatment or placebo. Importantly, the study incorporates a crossover design, allowing patients initially assigned to placebo to transition to active therapy after 2 months. This approach ensures that all participants have access to the investigational drug while preserving the rigor needed to assess treatment effect. Investigators aim to replicate the rapid and substantial reductions in blister burden observed in TAMES-01, reinforcing blistering as the key driver of disease severity in EBS.
The trial plans to enroll 40 patients, with an interim analysis scheduled after approximately 20 participants have completed treatment. If outcomes remain consistent with earlier findings, BioMendics intends to pursue breakthrough therapy designation and, pending regulatory alignment, advance directly into a pivotal phase 3 program. TolaSure has already received orphan drug and rare pediatric disease designations.
Beyond EBS, McGuire highlighted plans to expand TolaSure’s application to other rare keratinopathies. These include epidermolytic ichthyosis, driven by keratin 1 and keratin 10 mutations, and pachyonychia congenita, associated with keratin 6 and keratin 16 variants. Preclinical and early clinical work suggest that TolaSure’s autophagy-mediated mechanism of action is consistent across these disorders, supporting its potential as a platform therapy for multiple inherited diseases of keratin dysfunction.
TAMES-02 is actively recruiting at major centers of excellence, including Stanford University and Northwestern University, under the leadership of Joyce Teng, MD, PhD, and Amy Paller, MD. To minimize barriers to participation, BioMendics covers all travel-related expenses for enrolled patients. The company is also seeking engagement from dermatologists who care for larger EBS populations and may be interested in participating in future phase 3 studies.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.