Newly approved laser treatments can be an effective option for the treatment of this common skin disorder.

Newly approved laser treatments can be an effective option for the treatment of this common skin disorder.

Accure’s system is the first 1726nm-based laser device with both FDA and CE Mark clearances.
The use of laser-assisted drug delivery (LADD) is gaining momentum as the novel technique enables a more efficient delivery of drug to the lesion.

New Renuvion Dermal Handpiece is approved to effectively treat more dermal appearances such as wrinkles and rhytides.

AI’s ability to quickly process and interpret large quantities of health data is a valuable tool for physicians.

Many patients worry about privacy and accuracy.

As artificial intelligence use grows in the field of dermatology, and the potential of it getting integrated into the clinical practice becomes a reality, many may wonder how dermatologists feel about this advancement.

The skin tracking platform by Miiskin has added a new feature that allowed users to submit prescription requests virtually.
The results from the American Academy of Facial Plastic and Reconstructive Surgery annual survey showed an uptick in demand for facial plastic surgery, an increase in popularity for lifts and lasers, and a growing gender neutrality in patients.

In this week’s Pointers with Dr Portela, the 208SkinDoc highlights a growing issue of lasers made to look like or mimic the technology of already approved lasers but can have dangerous consequences.

FUE is a type of hair transplant that requires physicians to change tools to accommodate different hair and scalp types. Now, a recent study highlights a single device aiming to treat all types of hair situations.

The past year gave dermatologists ample cause for both celebration and frustration as they looked forward to new drugs, devices, and technology to optimize patient care. Here, Dermatology Times® editorial advisory board members weigh in on the good, bad, and still-TBD changes that shaped the specialty during 2021.

In this week’s Pointers with Dr Portela, the 208SkinDoc explains the differences between lasers that can be used for various treatment goals.

In this exclusive interview with Dermatology Times, David Goldberg, MD, JD, explains why lasers are a treatment option in acne and the latest advancements in the field.

In this exclusive video interview with Dermatology Times, Michael Gold, MD, tells us about his session at the New Frontiers in Cosmetic Medicine and Medical Dermatology Symposium.

The 11th annual New Frontiers in Cosmetic Medicine and Medical Dermatology Conference starts today. Here’s what to look forward to.

We sit down in a video interview with David Pariser, MD, FACP, FAAD, the secretary and founding member of the International Hyperhidrosis Society to discuss hyperhidrosis identifiers and treatments in honor of Hyperhidrosis Awareness Month.

R2 Technologies’ Novel Glacial Rx system has been cleared by the FDA for a new indication.

Jill Waibel, MD, discusses her recommendations on how to treat keloids and scaring using lasers in her presentation at the Skin of Color Update 2021.

A recent study delved into how well the 730 nm picosecond-domain laser could treat facial and non-facial lentigines in patients.

Part 2 of Dermatology Times®’ coverage of a strategic look at superficial radiation therapy (SRT) presented at the recent Music City SCALE Symposium for Cosmetic Advances & Laser Education 16th Annual Meeting details this therapy’s potential for reducing keloid recurrence.

Jason Emer, MD, in this video interview, discusses plasma pen cosmetic treatments, the influx of patient interest, dangers of non-FDA cleared pens, and how to work with SOC patients.

Cartessa Aesthetics, LLC launched Physiq, its hands free, noninvasive body contouring device.

Alma today announces the FDA clearance of its Alma Hybrid combination platform, which includes an ablative 10,600 nm (CO2) laser and nonablative 1570 nm laser for skin resurfacing.

The introduction of artificial intelligence into dermatology has been met with mixed emotions from the medical community. In this episode, Ade Adamson, MD, MPP, explains the benefits and detriments of the application, accuracy, and potential racial diagnostic bias.

At the final part of our Road Map to Recovery webinar recap series, sponsored by Aerolase, our expert panelist answered questions about vaccine mandates, social media marketing, cash balance planning, and more.

A recent study investigated the use of a microneedling radiofrequency device in improving skin laxity and fat deposits in patients.

Content-aware image restoration (CARE) technology demonstrated improvement in examining cellular details of skin.

Patients of color make up a growing market for aesthetic dermatology. Physicians must learn how to tailor treatments and assess skin of color risk to best serve this emerging patient population. Doing so will deliver positive outcomes and drive patient satisfaction.
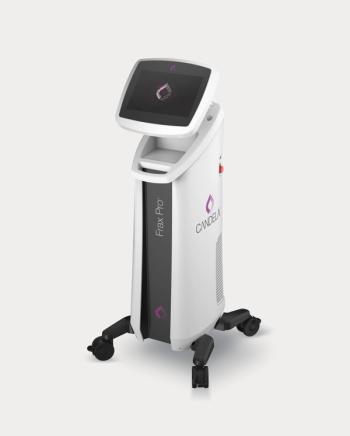
Candela announces the launch of its Frax Pro system, featuring dual-depth skin resurfacing with both novel Frax 1940 and Frax 1550 applicators.