Amgen releases positive phase 3 results evaluating U.S. FDA-approved apremilast (Otezla) for the treatment of mild-to-moderate plaque psoriasis in adults.

Amgen releases positive phase 3 results evaluating U.S. FDA-approved apremilast (Otezla) for the treatment of mild-to-moderate plaque psoriasis in adults.
The interleukin-23 antagonist guselkumab demonstrated greater efficacy compared with adalimumab and secukinumab at week 48 in controlled clinical trials investigating treatment of moderate-to-severe plaque psoriasis.
This week, we spoke with Mark Lebwohl, M.D., about his recent article featured in the Journal of the American Academy of Dermatology regarding biologic use in dermatology patients during the COVID-19 pandemic.

The latest American Academy of Dermatology-National Psoriasis Foundation phototherapy guidelines incorporate several advances in efficacy, safety and patient convenience that were unavailable a decade ago.
When it comes to biologic drugs, the latest joint American Academy of Dermatology-National Psoriasis Foundation guidelines are less prescriptive than descriptive, offering a comprehensive discussion on the facts of each biologic to inform physicians’ selections.

Dermavant announces the enrollment completion for their two phase 3 clinical trials investigating the topical therapeutic aryl hydrocarbon receptor (TAMA) tapinarof as a treatment for adult plaque psoriasis, with results from the study still expected in the second half of 2020 despite the current pandemic.

Brodalumab, guselkumab, ixekizumab and risankizumab stood out among 15 biologic and oral medications as having the highest short- and long-term response rates for the treatment of moderate-to-severe plaque psoriasis, according to a recent meta-analysis.
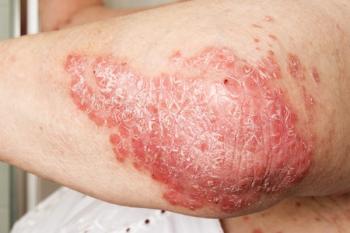
Current joint psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation emphasize the need to help patients understand the relationship between psoriasis and comorbid conditions and the importance of seeking appropriate interventions as needed.

Eli Lilly announces the U.S. Food and Drug Administration approval of ixekizumab (Taltz) for treatment of moderate-to-severe plaque psoriasis in pediatric patients 6 and under 18 who are also eligible for phototherapy and systemic therapy.

Dermatologists often combine multiple topical agents on their own, and have been doing so for many years. However, extemporaneous compounding of psoriasis topical agents may not necessarily yield what dermatologists anticipate.

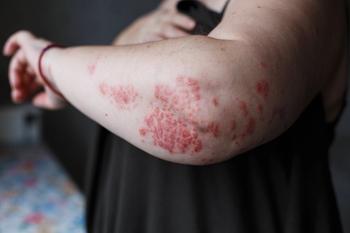
A study showing differences in baseline characteristics between responders to anti-tumor necrosis factor (anti-TNF) therapy and insufficient responders may be helpful to dermatologists as they consider treatment for patients with moderate-to-severe psoriasis, according to the investigators.
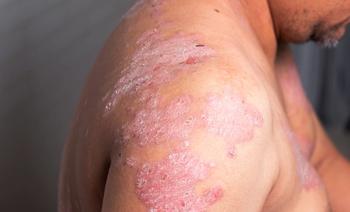
Switching patients with moderate-to-severe plaque psoriasis from one biologic to another is a multifactorial decision and may depend on safety, efficacy or dosing intervals. Ron Vender, M.D., offers insight into how to switch appropriately.

New joint AAD-NPF guidelines consider the quality-of-life impact of pediatric psoriasis as well as comorbidity risks in this patient population.

New topical treatments for psoriasis increasingly prove that vehicles matter, says an expert who spoke at the South Beach Symposium.

With extended remissions, high safety and favorable dosing schedules, interleukin (IL)-23 inhibitors are well-suited for a variety of patients with psoriasis, says an expert who spoke at the South Beach Symposium

Sol-Gel announces updates to their drug pipeline and their goals for 2020, which include submitting multiple provisional patents and NDAs for the treatment of a variety of skin conditions.
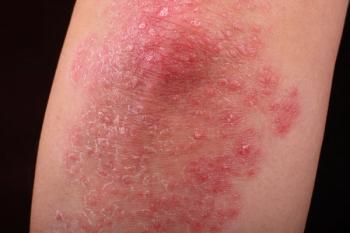
A Danish study suggests that TNF inhibitors may not increase the risk of developing recurring or new primary cancers in patients with inflammatory diseases.

Ustekinumab’s short-term effect on aortic vascular inflammation points to possible role of IL-12, IL-23 in cardiovascular disease, but more research is needed.

Pediatric patients with psoriasis who experience a significant improvement in disease severity after treatment may experience the greatest positive impact to quality of life, according to a recent study.

Risankizumab (SKYRIZI, AbbVie), outperformed secukinumab (Cosentyx, Novartis) in a head-to-head phase 3 study comparing the two treatments in adult patients with moderate to severe plaque psoriasis, according to data released by the company Jan. 14.
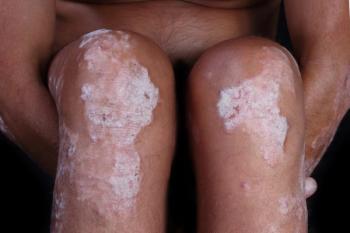
Topical halobetasol propionate 0.01% lotion appears to safely and quickly improve lower-extremity psoriatic lesions, according to a recent study.
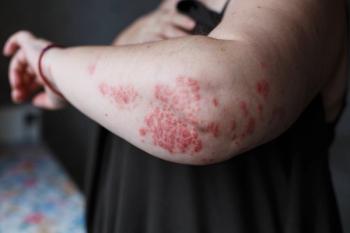
Results from an EU phase 3 clinical trial of a combination of betamethasone dipropionate and calcipotriene cream showed improved patient convenience, quality of life and efficacy compared to a betamethasone dipropionate and calcipotriene gel for patients with plaque psoriasis.

Newly published results from a phase 3 study demonstrate the superiority of bimekizumab over adalimumab for plaque psoriasis.

A recent study shows promising results for using ultrasound to examine hand tendon and joint inflammation in psoriatic arthritis patients.