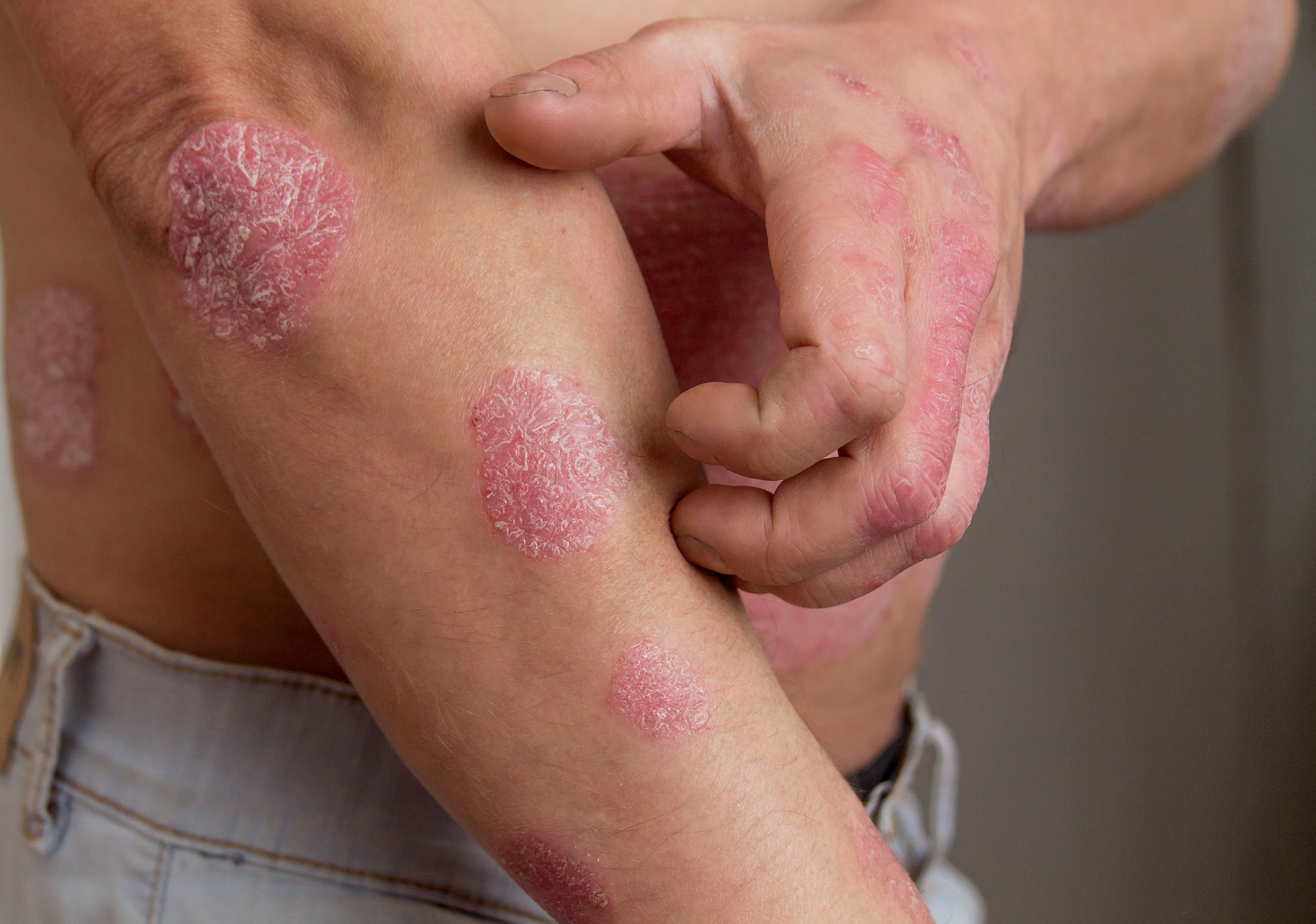
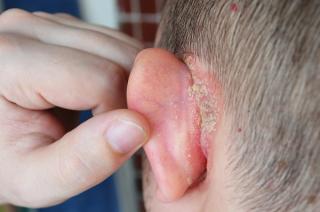
Psoriasis
Latest News
Latest Videos
CME Content
More News

Studies offer positive outcomes for patients with conditions like hidradenitis suppurativa or psoriasis.
A brief review of biologic agents being used or evaluated in the setting of plaque psoriasis.

Taking a broader look at moderate to severe plaque psoriasis, experts consider what the standard of care would be in different settings.

Skin sensitivity will be a factor in vehicles for new treatments.

The panel reviews therapeutic classes and their respective benefits in patients being treated for plaque psoriasis.

Centering discussion on a case of plaque psoriasis, experts share their experience with patient counseling and selecting therapy.

New research has been published in the New England Journal of Medicine on spesolimab as a treatment for generalized pustular psoriasis.
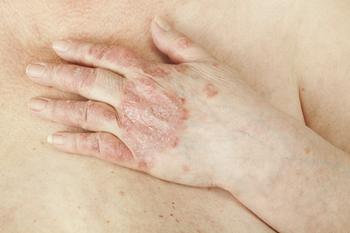
With this expanded indication, apremilast (Otezla; Amgen) is now the first and only oral treatment approved in adult patients with plaque psoriasis across all severities, including mild, moderate, and severe.

Considerations for the psychological and social impact that plaque psoriasis may have on a patient.

Panelists look deeper into the possible challenges or barriers to making an accurate diagnosis of plaque psoriasis

Arcutis has announced that the FDA has accepted the New Drug Application for roflumilast cream for adults and adolescent patients with plaque psoriasis.

A study in the Journal of the American Academy of Dermatology examined the incidence of psoriasis according to body mass index.

A recent study investigated apremilast use for psoriasis during COVID-19 infection.

Shared insight on identifying clinical manifestations of plaque psoriasis and making a timely diagnosis.

A panel of experts review the nature of plaque psoriasis and the mechanism of action of several classes of targeted therapy.

The FDA grants Priority Review for spesolimab (BI655130; Boehringer Ingelheim) as a treatment for generalized pustular psoriasis.

The New England Journal of Medicine has published the clinical trial data from the PSOARING 1 and PSOARING 2 studies on tapinarof treatment for plaque psoriasis.

In this exclusive video interview, James Q Del Rosso, DO, gets into the details of JAK inhibitors, explaining how they work and cutting through the headlines around the treatments.

In a video for Dermatology Times®, Mark Lebwohl, MD, details how to match patients with the right psoriasis treatment for their specific needs.
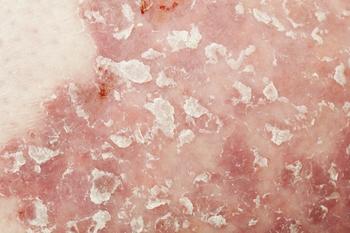
A phase 3 study evaluated apremilast as a treatment for patients with mild to moderate psoriasis.
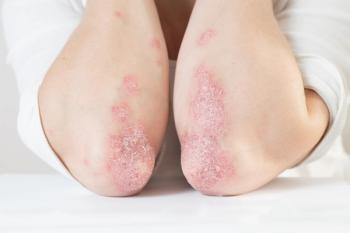
In this video interview, Mark Lebwohl, MD, explains why topicals for inflammatory conditions are vital for treatment.

In this episode, we dive into the background of the “The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas.” Adam Friedman, MD, and Misty Eleryan MD, MS, describe their process to creating this new dermatologic resource.

The study examines the impact of antirheumatic treatment on pregnancy outcomes in women with psoriatic arthritis.
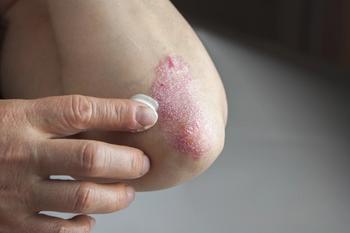
Mark G. Lebwohl, MD, presents on what dermatologists need to know about the newest psoriasis studies during the Fall Clinical Dermatology Conference.

Neal Bhatia, MD, explores steroid alternatives in his presentation at the Fall Clinical Dermatology Conference.