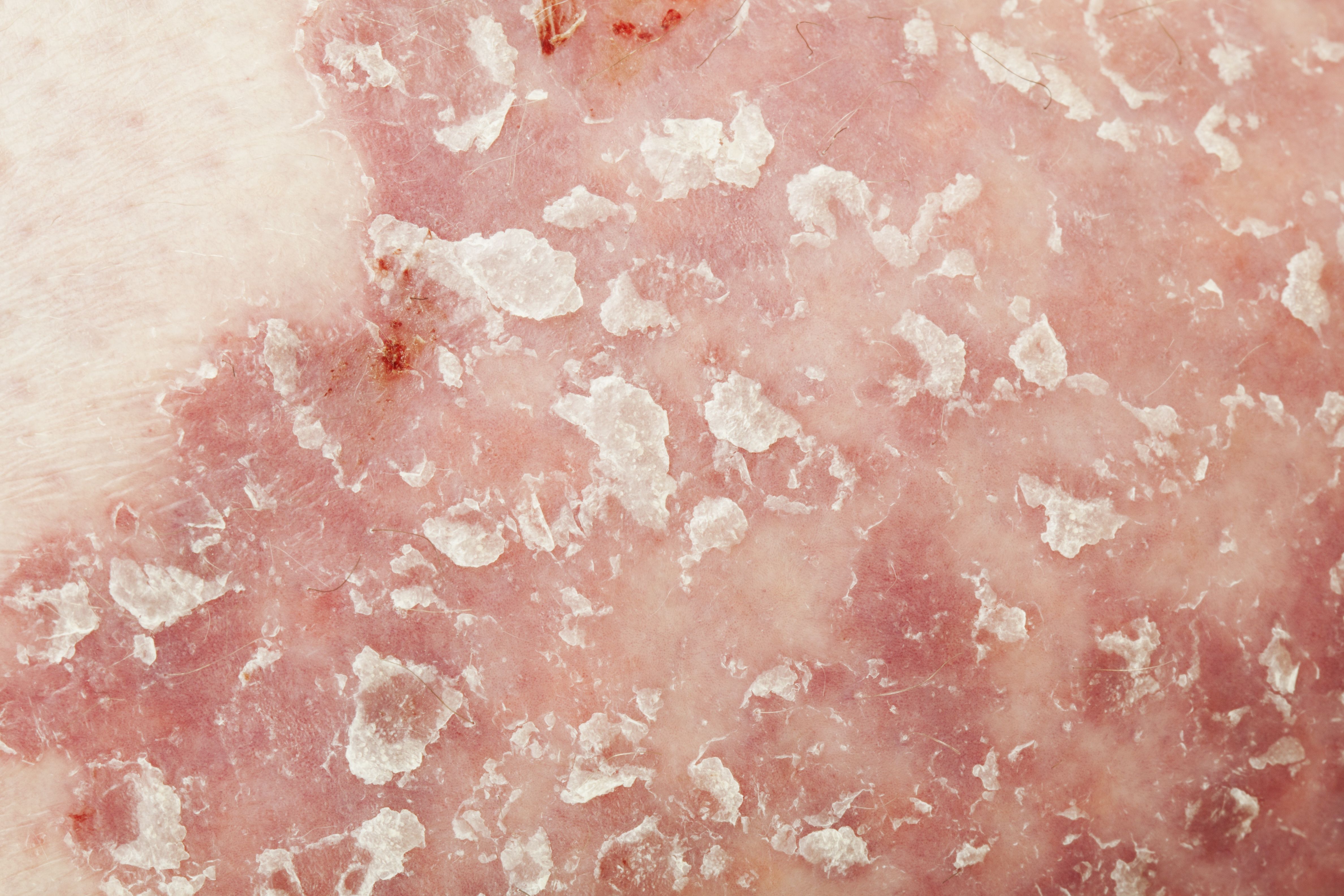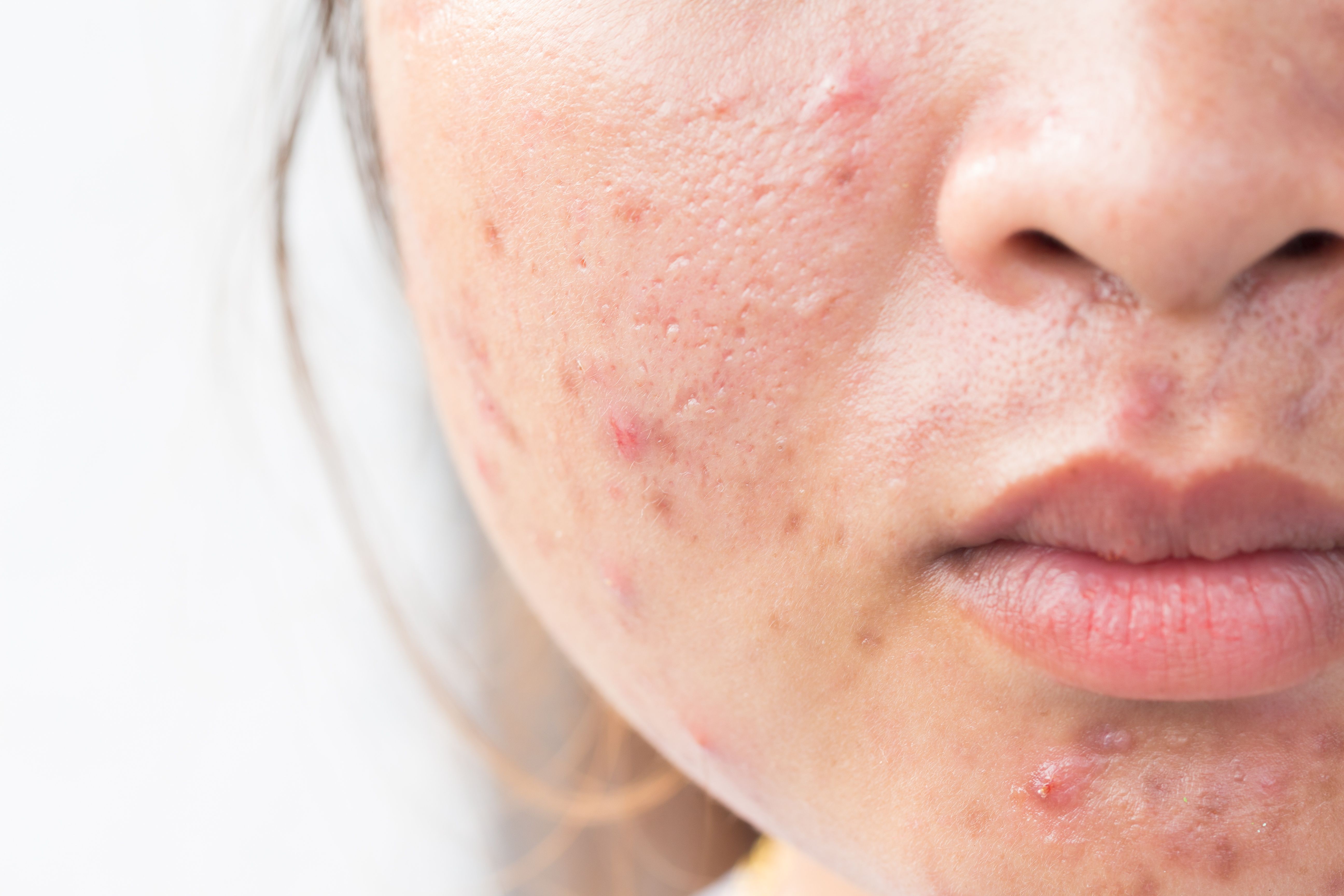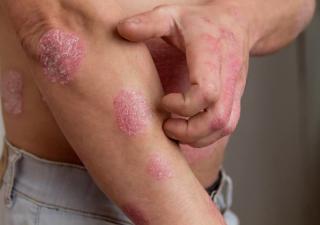
Psoriasis
Latest News
Latest Videos

CME Content
More News

At the opening session of the Society of Dermatology Nurse Practitioners Annual Symposium, Kathleen Haycraft, DNP, FNP-BC, DCNP, FAANP, delved into the revolutionary and evolutionary advances shaping dermatology in 2022.

In this video interview, Andrew Blauvelt, MD, MBA, dermatologist and clinical researcher at the Oregon Medical Research Center, highlights his key takeaways from his presentation at the Annual Society of Dermatology Nurse Practitioners Symposium, held April 22-23, in Nashville, Tennessee.

Arcutis Biotherapeutics announced news of its topical roflumilast foam’s trial on safety and efficacy of treatment in scalp and body psoriasis.

A recent study showed that men with psoriasis have larger EAT-V than those without the skin disease.

As innovation in dermatology continues, so do questions on the efficacy of cannabinoid use for psoriasis, among other skin diseases.

The results from the Evelo Biosciences EDP1815 treatment provide the first demonstration in a phase 2 trial of the safety and efficacy of an orally-administered, gut-restricted SINTAX medicine.

Nearly all survey takers reported wanting topical treatment options that are more effective, not steroids, and simpler to use.
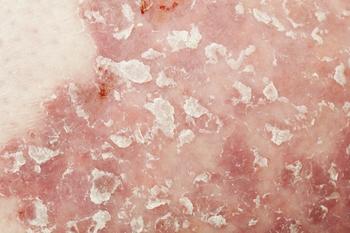
New data shows complete skin clearance using bimekizumab in moderate to severe plaque psoriasis open-label extension study.

The company announced the results from its long-term extension study, highlighting improved efficacy outcomes, quality of life, and tolerability data.

In a recent study from the Journal of the American Academy of Dermatology, investigators assessed symptoms, disease burden, and use of analgesics in patients with or without psoriatic arthritis.

A recent study examined the role of inflammation and psoriasis in cardiometabolic diseases, focusing on metabolic syndrome and its components.

The calcipotriene and betamethasone dipropionate cream is for the treatment of mild to moderate plaque psoriasis in adult patients, including the scalp.
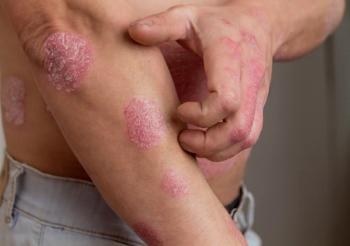
An article the in Journal of the American Academy of Dermatology examined the possible connection between psoriasis and colorectal cancer.

A recent study examined the link between psoriasis clearance, quality of life, and an uptick in cosmetic procedures.
Closing out their discussion on the management of plaque psoriasis, panelists share their excitement for future directions in care.

Experts reflect on the treatment of patients with plaque psoriasis during the COVID-19 pandemic.

A brief discussion on the unmet needs and ongoing challenges in the treatment of plaque psoriasis.

Shared insight on which factors are most important in determining second-line therapy for plaque psoriasis.

Linda Stein Gold, MD, discusses psoriasis in 2022, in her presentation at the current Maui Derm for Dermatologists meeting.

New and exciting topical and oral therapies for psoriasis have changed the way dermatologists treat and manage their psoriasis patients.
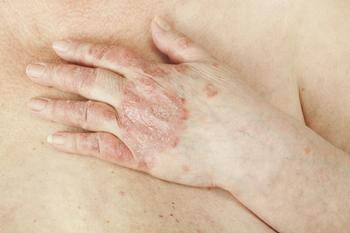
Neal Bhatia, MD, highlights the most exciting new drugs and therapies in atopic dermatitis and psoriasis in his presentation at the current Maui Derm for Dermatologists meeting.

Moving on to the second patient case, panelists discuss switching therapy at treatment failure for plaque psoriasis.

Panelists share which factors they use to determine optimal therapy in the moderate to severe plaque psoriasis setting.

A research letter published by the Journal of the American Academy of Dermatology dove into the question of how psoriasis may increase the overall risk of cutaneous malignant melanoma in patients.

A study published in the Journal of the American Academy of Dermatology did a prospective analysis on psoriasis biologic treatment response related to comorbid obesity and a history of diabetes.