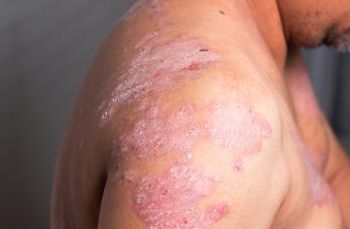
A study finds low risk of malignancy from secukinumab treatment for psoriasis, psoriatic arthritis and ankylosing spondylitis patients, despite limited data.
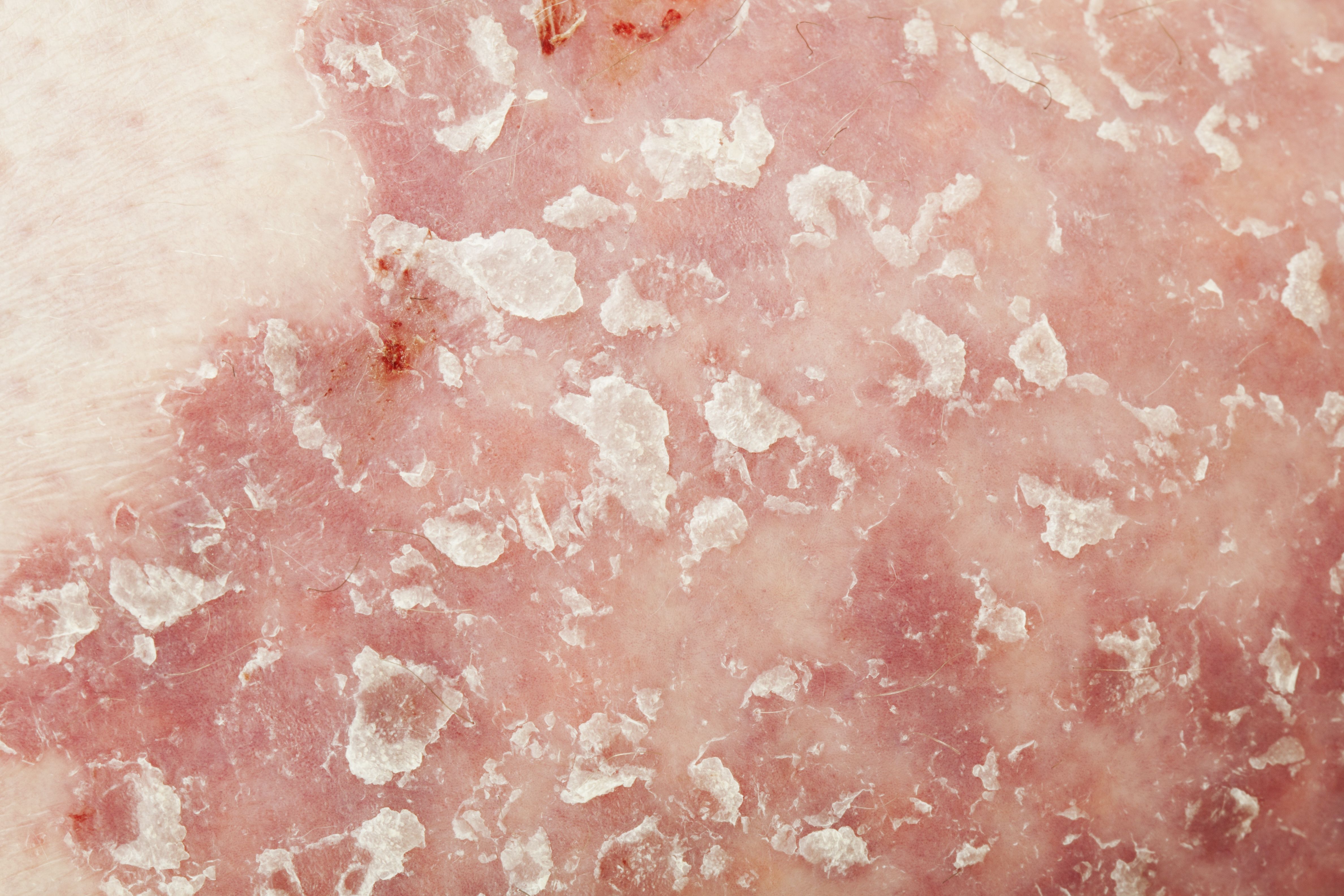

A study finds low risk of malignancy from secukinumab treatment for psoriasis, psoriatic arthritis and ankylosing spondylitis patients, despite limited data.
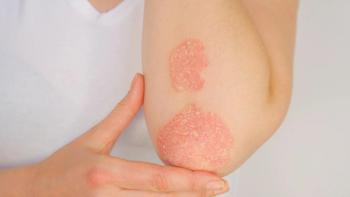
AbbVie announces the submission of regulatory filings for risankizumab to the FDA and EMA seeking approval for the drug as a treatment for psoriatic arthritis.

A study found psoriasis patients treated with biologic therapy had a significant reduction in high-risk plaque in heart arteries over 1 year.
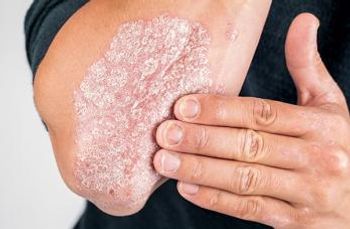
A new study in Arthritis Care & Research found depression and anxiety reduces the possibility of achieving minimal disease activity in patients with psoriatic arthritis.
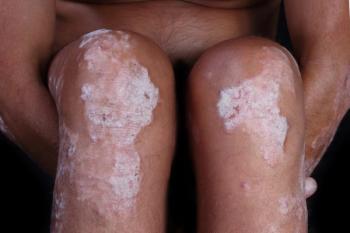
Sun Pharmaceuticals announces the publication of results from its 5-year extension studies evaluating the safety, efficacy and tolerability of tildrakizumab-asmn.

Pre- and postmarketing psoriasis studies support novel drug results and new labeling.

Dermavant announces positive results from an interim analysis of its PSOARING 3 safety study investigating tapinarof cream 1% for the treatment of plaque psoriasis in adult patients.

According to one expert, recently completed phase 3 trials of novel drugs have shown impressive results, with 2021 expected to be an exciting year that will bring further advances in treatment options for patients with moderate-to-severe plaque psoriasis.
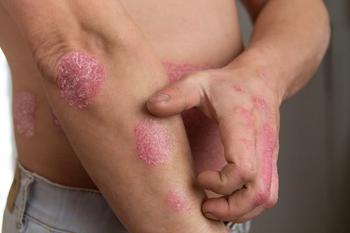
New AAD-NPF guidelines focus on biologics, topical therapy, systemic non-biological therapies, management of pediatric patients, use of phototherapy and comorbidities.

A proprietary food supplement designed to balance the gut microbiome and target the over production of skin cells will be examined in an upcoming consumer study that has recently announced participant enrollment.

Safety profiles remain consistent longer-term but cost and accessibility are major challenges for biologics aimed at treating psoriasis.

New guidance statements related to COVID-19 vaccines and psoriatic disease patients have been published by the National Psoriasis Foundation (NPF) COVID-19 Task Force.
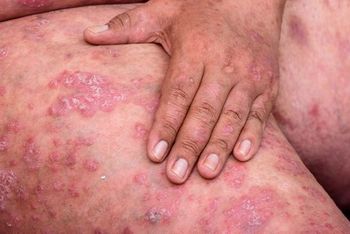
Several systemic therapies are currently available for the treatment of patients with moderate-to-severe psoriasis. Biologics changed the game for treatment. Continued study of and innovation in IL-17 and IL-23 inhibitors promise even better results, but clinicians need to understand each biologic in detail.

Several systemic therapies are available for the treatment of patients with moderate to severe psoriasis. Continued research has further elucidated the immunopathogenesis of the disease, leading to the development of novel biologic agents that are proving to more effectively and safely address moderate to severe psoriatic lesions.

According to a study conducted by Galderma, both rosacea and psoriasis have a significant QOL impact on patients, with a proportion of patients reporting feelings of anxiety and depression due to their disease.

A recent study found patients with high baseline cardiovascular risk who had taken ustekinumab were at increased risk for developing severe cardiovascular events following treatment.

Highlighting the relationship between long-term safety and drug survival, a recent review may help address physician reluctance in prescribing conventional systemic psoriasis treatments.

Genetic polymorphisms may increase psoriasis susceptibility, according to a study that found patients who have the G allele in the JAK1 gene have twice the risk of developing psoriasis, and for those with the JAK3 allele, the risk rises nearly 2-fold.

In a recent study, psoriasis drugs showed widely variable drug survival rates, owing to differences in safety, efficacy, patient satisfaction and other factors. However, concerns linger over potential to cause long-term cumulative organ toxicity.

Comorbidities, co-medication, organ impairment, functional deterioration and frailty make treatment plans challenging for older psoriasis patients. However, these patients should not be precluded but will require more extensive evaluation and assessment, according to a recent study.

Studies confirm improvement achieved and maintained through one year of active treatment with similar scores for patients switching from placebo to guselkumab.

Ixekizumab demonstrated long-term efficacy in treating psoriasis and psoriatic arthritis across five years, according to data presented by Eli Lilly at the 29th annual European Academy of Dermatology and Venereology (EADV) Congress.
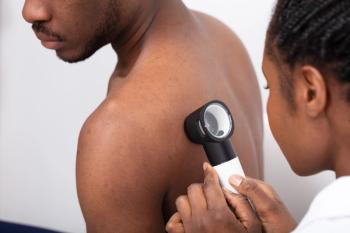
A majority of psoriasis patients in phase 3 clinical trials for biologics are white, says authors of a recent study. As ethnicity and race may play a role in response to biologics, researchers emphasize need for more diversity in clinical trials.
Ardon Health patients with autoimmune conditions have expressed common questions and concerns about COVID-19.

A phase 3b study investigating the efficacy and safety of risankizumab compared to secukinumab showed positive results, with risankizumab demonstrating noninferiority to secukinumab at week 16, and superiority at week 52.