- Dermatology Times, October 2025 (Vol. 46. No. 10)
- Volume 46
- Issue 10
Cutaneous Toxicities From Breast Cancer Therapies: What Dermatologists Need to Know
Key Takeaways
- Cutaneous toxicities from breast cancer therapies can lead to treatment interruptions and quality-of-life impairments, with alopecia being a major concern for patients.
- Dermatologists are essential in managing dermatologic adverse effects (dAEs) from both traditional and targeted therapies, improving patient outcomes.
Explore the critical role of dermatologists in managing cutaneous toxicities from breast cancer therapies, enhancing patient quality of life and treatment outcomes.
Cutaneous toxicities from anticancer therapies are common across breast cancer regimens and can lead to impactful dose reductions, treatment interruptions or cessation, and quality-of-life and psychosocial impairments.1 Hair loss, in particular, is reported as the most feared complication of breast cancer treatment in more than 70% of patients,2 with nearly 10% considering refusal of chemotherapy due to potential hair loss.3 Almost half of patients describe alopecia as the most distressing aspect of chemotherapy.
A dermatologist’s familiarity with the varied and common dermatologic adverse effects (dAEs) of both targeted therapies and traditional chemotherapies is essential for optimizing patient outcomes. Although immunotherapy and antibody-drug conjugates (ADCs) are being used with increased frequency and with new associated toxicities, understanding common patterns of both old and new drugs is critical in determining likely drug culprits and managing AEs. Both patients with breast cancer and survivors are treated by dermatologists across the entire clinical care and practice continuum. Herein, we discuss the most common dAEs in patients with breast cancer and offer practical clinical pearls for prevention and symptom management.
Traditional Cytotoxic Chemotherapy
Despite advances in targeted therapy, cytotoxic chemotherapies remain central to both localized and metastatic breast cancer treatments. Anthracyclines (doxorubicin, epirubicin) and taxanes (paclitaxel, docetaxel, nab-paclitaxel) are standard for high-risk early disease; other agents include alkylating agents (cyclophosphamide), platinum-based drugs (carboplatin, cisplatin), antimetabolites (5-fluorouracil [5-FU], capecitabine, gemcitabine), and vinca alkaloids (vinorelbine). By disrupting cell proliferation, these agents frequently and nonspecifically damage rapidly dividing cells of the hair, skin, nails, and mucous membranes.4
Chemotherapy-Induced Alopecia. One of the most common dAEs of breast cancer cytotoxic chemotherapies, including anthracyclines, taxanes, platinum agents, antimetabolites, and vinca alkaloids, is chemotherapy-induced alopecia (CIA), or anagen effluvium.5 Cited CIA rates approach 80% after taxane therapy, 100% after doxorubicin, and 60% after alkylating agents. Combination regimens are common for breast cancer treatment and may incur even higher rates.6
With many regimens, scalp cooling therapy can meaningfully reduce the incidence of CIA in the majority of patients and does not increase the risk of scalp metastasis.3,7,8 Knowledge of the likelihood of hair retention based on regimen is helpful in counseling patients (eg, nearly all patients undergoing taxane monotherapy and scalp cooling therapy retain sufficient hair to avoid a wig).8 Importantly, cooling therapy also decreases the risk of permanent CIA, a rare but highly distressing complication of cancer treatment. Due to demonstrated CIA impact on quality of life and psychological well-being, knowledge of scalp cryotherapy and referral to scalp cooling centers is an essential role of the dermatologist.9 The Scalp Cooling Study Library10 is an online resource that includes a scalp cooling center registry, CIA risk calculator, and a scientific literature repository.
Hand-Foot Syndrome. Anthracyclines, taxanes, antimetabolites (5-FU, capecitabine), and rarely vinca alkaloids can cause painful erythematous, desquamating eruptions more commonly localized to the palms than the soles. This is known as hand-foot syndrome (HFS), or palmoplantar erythrodysesthesia, which is a variant of toxic erythema of chemotherapy.5,11 Incidence with cytotoxic chemotherapy approaches 60% and can impair activities of daily living (ADLs). Certain regimens—particularly taxanes and doxorubicin—are highly responsive to cooling as effective prophylaxis. Frozen gel gloves and socks (or ice packs over a thin glove/sock) applied 15 minutes before, throughout, and after infusion can significantly mitigate HFS and nail toxicity. Once present, HFS management may include oral analgesics, high-potency topical steroids, diclofenac gel, and oral steroids in severe cases with bullae or bleeding. High-dose vitamin D (50,000-200,000 international units [IU]) can be very effective in acute, severe cases of toxic erythema (including HFS) and maintains safety up to 600,000 IU even in ill, hospitalized patients.12 Celecoxib is uniquely effective for capecitabine-induced HFS.11
Dorsal HFS. Taxane-based regimens may cause an HFS variant characterized by erythema of the dorsal hands more commonly than the feet, Achilles tendon, and/or malleoli. Dorsal HFS is unique to taxanes and is associated with nail toxicities (onycholysis, paronychia, onychomycosis, Beau lines). Prophylactic cryotherapy, as above, is highly effective for dorsal HFS, nail involvement, and quality-of-life preservation.5
Nail Changes. Nail toxicity is most common with taxanes and anthracyclines but is also seen with alkylators and immunotherapy. Nail changes include onycholysis, erythronychia, subungual hemorrhage or abscess, melanonychia, Beau lines, and leukonychia.11 Nail toxicity can be painful, predispose to infection, limit ADLs, and interrupt treatment. Prophylactic cryotherapy, as above, is particularly effective in taxane-related disease.13 Nail trimming, dilute vinegar soaks (1:15), and topical antiseptics help prevent trauma and more significant infection.11
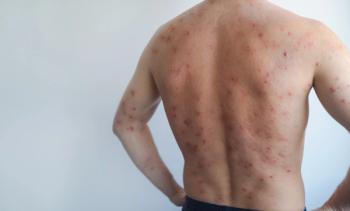
Morbilliform Rash.True morbilliform eruptions are common across multiple anticancer classes and most commonly represent a delayed-type hypersensitivity (DTH). Importantly, a skin exam to rule out more specific diagnostic features of toxicity is critical. Although taxanes and their preservatives are a common cause of DTH following typical timing (appearance 4-14 days after drug initiation with more rapid time to onset with subsequent doses), they also induce dorsal HFS, inflame actinic keratoses (AKs), and may cause local eruptions in areas that are heated during treatment (eg, warming blankets, heated infusion chair). The exam of a patient with taxane toxicity might include inflamed AKs of the V-neck, upper back, and face, or taxane-related dorsal HFS, but would lack typical morbilliform changes expected on the sun-protected trunk and limbs. This patient could be safely managed with topical steroids, heat avoidance/cooling during infusion, and expectant management.
In the case of a true morbilliform eruption, mild, topical corticosteroids and antihistamines for associated pruritus are often sufficient. Additionally, clinical and laboratory evaluation for severe cutaneous adverse reaction is always warranted, keeping in mind that oral mucositis may be a direct toxic effect of therapy. Mucosal involvement warrants a thorough exam of other sites.11 Importantly, in darker skin tones, morbilliform rashes may be subtle or appear violaceous, not overtly erythematous.
Mucositis. Oral mucositis is common with doxorubicin and may be seen with alkylating agents and taxanes in patients with breast cancer. This typically spares nonkeratinized tissues of the hard palate and dorsal tongue. Good oral hygiene reduces infection risk. Treatment includes dexamethasone mouth rinse (0.1 mg/mL) or clobetasol gel (0.05%); severe cases may require intralesional or systemic steroids. Pain can be managed with morphine or benzydamine mouthwash, topical/oral analgesics, or low-level laser therapy. Oral cryotherapy (ice chips) during infusion is beneficial. Glutamine and honey have some evidence of benefit, whereas chlorhexidine, sucralfate, and chewing gum should be avoided.11
Hyperpigmentation. Skin hyperpigmentation is common across multiple drug classes, including alkylating agents (especially of palms and soles), cyclophosphamide (oral mucosa), platinum agents (localized, patchy dyspigmentation), 5-FU (varied), capecitabine (acral pigmentation), and taxanes (serpentine supravenous hyperpigmentation, particularly at docetaxel infusion sites). Hyperpigmentation typically resolves after therapy and should not disrupt treatment. Though there is no specific management, this can be distressing, especially in patients with darker skin types; sun protection can help limit severity in some cases.5
Hypersensitivity Reactions. Type I hypersensitivity reactions (HSRs) are most associated with platinum agents (commonly between cycles 4 and 8) and can be managed with desensitization protocols and prophylactic steroid/antihistamine premedication. HSRs are also observed in HER2-targeted therapies and are further described in the following section.11
Radiation Recall Dermatitis.Radiation recall can be triggered by many antineoplastic agents but are classically triggered by traditional cytotoxic chemotherapy, including anthracyclines, taxanes, and antimetabolites. Radiation recall can occur years after low doses of radiation. Morphology consists of sunburn-like dermatitis with erythema, edema, desquamation, vesiculation, and/or ulceration, with associated pain and pruritus. Preinfusion treatment or dose reduction in severe cases can help prevent recurrence.14
ADCs, HER2 Monoclonal Antibodies, and HER2 Tyrosine Kinase Inhibitors
ADCs. Cutaneous toxicities of trastuzumab deruxtecan (T-DXd), ado-trastuzumab emtansine (T-DM1), and sacituzumab govitecan (SG) are generally mild, resembling chemotherapy effects such as alopecia, morbilliform eruptions, and aphthous stomatitis. Alopecia occurs in up to 40% of patients on T-DXd and SG. T-DM1 causes fewer dAEs due to its noncleavable linker but has been associated with cutaneous and mucosal telangiectasias.1,15 Management parallels chemotherapy-induced toxicities; scalp cooling may help, and there are ongoing clinical trials to study this.15
Anti-HER2 monoclonal antibodies. Trastuzumab and pertuzumab can trigger EGFR-like toxicities, likely due to HER2/HER1 (EGFR) dimerization. This most often includes papulopustular (acneiform) rash. Prophylaxis includes moisturizers, sunscreen with high sun protection factor, topical steroids, and oral tetracyclines. Other EGFR-associated effects, including photosensitivity, xerosis with pruritus, staphylococcal superinfection, and hair/nail changes, are less frequent due to relative HER2 specificity.11,16
Up to 40% of patients on HER2 antibodies or ADCs develop HSRs, often after multiple uneventful infusions. Presentations include urticaria, pruritus, flushing, abdominal cramping, and anaphylaxis. Twelve-step desensitization protocols enable continuation, and premedication with antihistamines, leukotriene blockers, and corticosteroids helps prevent recurrence. Type I HSRs from platinum alkylating agents are managed similarly.11,17
HER2 Tyrosine Kinase Inhibitors. Agents such as tucatinib, lapatinib, and neratinib (often combined with trastuzumab or capecitabine) cause fewer dAEs than other classes. When present, they resemble chemotherapy toxicities. HFS occurs in up to 17% with lapatinib, along with nail changes and dyspigmentation.18 Management is consistent across agents.
Endocrine Therapies
Hormone-modifying therapies include aromatase inhibitors (AIs; letrozole, anastrozole, exemestane), selective estrogen receptor modulators (SERMs; tamoxifen), and selective estrogen receptor degraders (SERDs; fulvestrant, elacestrant). Alopecia is most often linked to AIs and less commonly SERMs via androgenic modulation, presenting as frontoparietal thinning similar to androgenetic alopecia.9 Scalp cooling is ineffective, but topical 5% minoxidil twice daily can promote regrowth, and low-dose oral minoxidil (1.25-2.5 mg daily) is increasingly utilized. Additionally, oral spironolactone (≤ 200 mg/day) may be considered cautiously due to theoretical receptor stimulation.9 Although SERDs rarely cause dAEs alone, alopecia has been reported in fulvestrant-based combination regimens with CDK, PI3K, and AKT inhibitors.19 Management includes topical 5% minoxidil or low-dose oral minoxidil when CDK4/6 inhibitors are implicated.20 SERD-CDK regimens may also cause stomatitis and morbilliform rash.21
Other hormone therapy–related dAEs include flushing and immunologic drug eruptions, most commonly morbilliform rash in up to 10% of patients. Rare reports include subacute cutaneous lupus erythematosus (SCLE), leukocytoclastic vasculitis, erythema nodosum, urticaria, eczematous or erythema multiforme–like eruptions, scleroderma-like reactions, Sweet syndrome, radiation recall dermatitis, and Stevens-Johnson syndrome/toxic epidermis necrolysis. Management generally involves topical/oral steroids with antihistamines; therapy should be held in severe cases, with resolution typically within 1 to 2 weeks. Rechallenging with the same or an alternative drug is often feasible. SCLE warrants drug hold; after rheumatologic evaluation for systemic disease, patients can often continue the anticancer regimen with topical steroids, strict UV protection, and consideration of hydroxychloroquine. Erythema nodosum may be treated with steroids, nonsteroidal anti-inflammatory drugs, colchicine, or dapsone without the need for therapy discontinuation.11
Of note, hormone therapy–associated dAEs often appear 1 to 2 months after treatment initiation.11
Immune Checkpoint Inhibitors (ICIs) and ICI Combination Regimens
Cutaneous toxicities from immunotherapies or combination regimens (eg, nab-paclitaxel plus atezolizumab) are common, occurring in up to two-thirds of patients on some combination regimens, and often appear early in the treatment course. Specific dermatologic manifestations most often include pruritus, eczematous dermatoses, morbilliform eruptions, lichenoid dermatoses, psoriasis, vitiligo, and bullous pemphigoid (BP). Infrequently, cutaneous sclerosis, dermatomyositis, cutaneous lupus, and vasculitides have been observed; these rarer presentations are typically treated similarly to non–ICI-induced disease.1
Pruritus is among the most common immune-related AEs (irAEs) and is effectively managed with a step-up approach including emollients and antihistamines with graduation to doxepin, gabapentin/pregabalin, naloxone, mirtazapine, or aprepitant based on patient-specific factors. Non-BP should be considered.
Eczematous eruptions can have atopic, nummular, or contact dermatitis–like presentations. A combination of topical steroids and antihistamines is effective for mild cases, whereas phototherapy and dupilumab can be employed for more extensive disease. Importantly, the use of dupilumab in solid tumor ICI recipients is not associated with worse cancer outcomes.22
Morbilliform eruptions are more often encountered in CTLA-4 inhibitor regimens, are typically self-limited, and can be managed with emollients, antihistamines, and topical steroids. When present and due to ICI, they are frequently mild and transient. In patients with breast cancer undergoing combination therapy with carboplatin/paclitaxel and pembrolizumab,23 the morbilliform eruption between days 7 and 14 is almost always due to the taxane, whereas the rash is almost always incorrectly attributed to the ICI.
Lichenoid dermatoses are frequently associated with PD-1/PD-L1 inhibitors and present as violaceous, pruritic papules/plaques with or without oral involvement. Topical steroids are often sufficient, whereas phototherapy, acitretin, dupilumab, and hydroxychloroquine can be added for moderate to severe cases. Although dupilumab has been studied, the impact of hydroxychloroquine on cancer outcomes, if any, is not yet known.
Psoriasiform eruptions can present as flares of existing (usually within 4-6 weeks) or de novo psoriasis (typically at 3-6 months) of any morphologic subtype and/or psoriatic arthritis (PSA). Topical steroids and vitamin D analogues are first line; acitretin is particularly useful when there is lichenoid overlap. Apremilast is favored with palmoplantar involvement or concomitant oral ulceration but avoided with concomitant colitis due to diarrhea as an AE. Anti-TNF-α or IL inhibitor biologics are recommended in more extensive cases or with coincident arthritis. In the absence of PSA, and particularly with concomitant colitis, IL-23 inhibitors are favored. With arthritis, IL-17 or IL-23 inhibitors are effective, with a preference for IL-17 inhibitors in the setting of erosive or axial PSA. Studies of the impact of targeted biologic therapy for psoriasis/PSA are ongoing.
Vitiligo is most common in patients with melanoma, may indicate improved ICI response, and requires no specific treatment apart from UV avoidance counseling, though typical therapies may be effective.
BPoccurs with increased frequency in ICI-treated patients, though it does occur more commonly in older men. Importantly, prebullous disease should be considered in refractory itch. Targeted approaches, including dupilumab, rituximab, and omalizumab, are effective and safe.24 Dapsone, methotrexate, intravenous immunoglobulin, and plasma exchange have all been used based on severity.1 Although tetracyclines may be effective in some cases, their impact on gut microbiome diversity and its impact on ICI outcomes should be considered.
Importantly, for all cutaneous irAEs, systemic steroids are rarely required and reserved for high-grade eruptions due to potential interference with ICI-mediated tumor control. Systemic steroids should be avoided in almost all cases of psoriasis and PSA due to risk of flare upon withdrawal, lack of long-term efficacy, and risk of impaired cancer outcomes.1
Other Targeted Therapies
CDK and PARP Inhibitors.Cutaneous toxicities from CDK inhibitors (abemaciclib, palbociclib, ribociclib) are uncommon and usually mild, presenting as xerosis with pruritus and managed with supportive care. Combinations of CDK4/6 inhibitors and endocrine treatment may cause androgenetic alopecia and can be managed with topical or oral minoxidil.1,20 PARP inhibitors (olaparib, talazoparib) rarely cause HFS. Other reported events include erythema nodosum, pseudoporphyria, urticaria, Sweet syndrome, photosensitivity, and morbilliform eruptions.20
PI3K Inhibitors. Alpelisib and inavolisib (for hormone receptor [HR]+/HER2–, PIK3CA-mutated disease) are associated with morbilliform or urticarial-like rashes that usually fade within 1 to 2 weeks. These respond to topical steroids and antihistamines; prophylactic antihistamines aid in prevention and drug reintroduction. Inavolisib appears to have lower rash incidence.1,20
AKT Inhibitors. Capivasertib (approved in 2023 with fulvestrant for advanced HR+/HER2– disease) can induce morbilliform eruptions in up to 56% of patients, with 15% being at least grade 3. Mild rash is manageable with topical steroids without drug interruption need. Severe cases require systemic corticosteroids and treatment cessation if possible. Rechallenge may be tolerated.20,25
Radiation Therapy
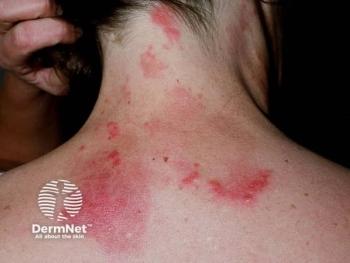
Radiation dermatitis (RD) can be acute (< 90 days post treatment) or chronic (> 90 days). Acute RD manifests as erythema, xerosis, hyperpigmentation, desquamation, or bullae. Chronic RD presents with atrophy, fibrosis, edema, telangiectasias, necrosis, or ulceration. Complications include secondary infection (acute) and increased nonmelanoma skin cancer risk (chronic).
Importantly, acute RD can be minimized by washing with gentle cleansers and water and applying a midpotency cream-based topical steroid throughout the radiation therapy course.26 Patients should apply the steroid after treatment and before bed. This approach should be avoided in patients with inflammatory or very superficial breast cancer, where damage to the skin is necessary for appropriate radiation dosing. Mild, acute RD is managed with bland moisturizers, loose clothing, and midpotency topical steroids. Severe cases may require high-potency steroids, topical antibiotics, or debridement. In chronic RD, pulsed dye laser can minimize telangiectasias. Vitamin E with pentoxifylline (200-400 mg every 8 hours) may help with sclerosis and radiation therapy–associated morphea. Skin exams with palpation are recommended for secondary malignancy screening.11
Conclusion
Dermatologists can play a critical role in supporting patients with breast cancer through the entire treatment life cycle. Knowledge of common and likely AEs with appropriate counseling and intervention allows for optimized symptom control, quality-of-life improvement, and prevention of unncessary treatment dose reduction, interruption, or cessation.
Nicole R. LeBoeuf, MD, MPH, is vice chair of the department of dermatology at Dana-Farber, chief of oncodermatology at Brigham and Women’s Hospital, and associate professor at Harvard Medical School in Boston.
Grace B. Hanrahan, is a third-year medical student at the University of Massachusetts T.H. Chan Medical School in Worcester, Massachusetts.
References
- Anders CK, LeBoeuf NR, Bashoura L, Faiz SA, Shariff AI, Thomas A. What’s the price? toxicities of targeted therapies in breast cancer care. Am Soc Clin Oncol Educ Book. 2020;40:55-70. doi:10.1200/EDBK_279465
- Haksöyler V, Koseci T, Olgun P, Bayram E Çaparlar MA. Effectiveness and reliability of scalp cooling in preventing hair loss caused by chemotherapy. Kocaeli Med J. 2021;10(2):133-140. doi:10.5505/ktd.2021.53325
- Kadakia KC, Rozell SA, Butala AA, Loprinzi CL. Supportive cryotherapy: a review from head to toe. J Pain Symptom Manage. 2014;47(6):1100-1115. doi:10.1016/j.jpainsymman.2013.07.014
- Deutsch A, Leboeuf NR, Lacouture ME, McLellan BN. Dermatologic adverse events of systemic anticancer therapies: cytotoxic chemotherapy, targeted therapy, and immunotherapy. Am Soc Clin Oncol Educ Book. 2020;40:485-500. doi:10.1200/EDBK_289911
- Anoop TM, Joseph PR, Pn M, Kp P, Gopan G, Chacko S. Cutaneous toxicities in breast cancer patients receiving chemotherapy and targeted agents—an observational clinical study. Clin Breast Cancer. 2021;21(4):e434-e447. doi:10.1016/j.clbc.2021.01.009
- Alizadeh N, Mirpour SH, Darjani A, Rafiei R, Rafiei E, Mohammadhoseini M. Dermatologic adverse effects of breast cancer chemotherapy: a longitudinal prospective observational study with a review of literature. Int J Dermatol. 2020;59(7):822-828. doi:10.1111/ijd.14916
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317(6):596-605. doi:10.1001/jama.2016.20939
- Smetanay K, Junio P, Feißt M, et al. COOLHAIR: a prospective randomized trial to investigate the efficacy and tolerability of scalp cooling in patients undergoing (neo)adjuvant chemotherapy for early breast cancer. Breast Cancer Res Treat. 2019;173(1):135-143. doi:10.1007/s10549-018-4983-8
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80(5):1199-1213. doi:10.1016/j.jaad.2018.03.056
- The Scalp Cooling Study Library. Accessed September 12, 2025. https://scalpcoolingstudies.com/
- LeBoeuf NR, Alloo A. Cutaneous Toxicities From Anticancer Therapies. Wolters Kluwer; 2023.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin D for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159(2):219-222. doi:10.1001/jamadermatol.2022.5397
- Scotté F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23(19):4424-9. doi:10.1200/JCO.2005.15.651
- Sibaud V, Lebœuf NR, Roche H, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. 2016;26(5):427-443. doi:10.1684/ejd.2016.2833
- Gronbeck C, Hadfield MJ, Grant-Kels JM. Dermatologic toxicities of antibody-drug conjugates. J Am Acad Dermatol. 2024;91(6):1177-1188. doi:10.1016/j.jaad.2024.08.036
- Sheu J, Hawryluk EB, Litsas G, Thakuria M, LeBoeuf NR. Papulopustular acneiform eruptions resulting from trastuzumab, a HER2 inhibitor. Clin Breast Cancer. 2015;15(1):e77-e81. doi:10.1016/j.clbc.2014.09.003
- Kempke AP, Kraft S, Castells M, Ravikumar R, Van Poznak C. Desensitization permits continued trastuzumab and ado-trastuzumab emtansine therapy after hypersensitivity reaction. J Hematol Oncol Pharm. 2020;10(6):380-383.
- Tang X, Wang C, Li Y, Tang J, Zhang G, Chen L. Signal detection and safety analysis of three tyrosine kinase inhibitors for HER-2 positive breast cancer: a retrospective study based on the FAERS database. Front Pharmacol. 2025;16:1538881. doi:10.3389/fphar.2025.1538881
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997-3005. doi:10.1016/S0140-6736(16)32389-3
- LeVee A, Deutsh A, Lindgren ES, et al. New drugs, new toxicities: side effects of new and emerging breast cancer therapies. Am Soc Clin Oncol Educ Book. 2025;45(3):e473384. doi:10.1200/EDBK-25-473384
- Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116-124. doi:10.1001/jamaoncol.2019.4782
- Khattab S, Wan G, Xu S, et al. Long-term mortality outcomes among immunotherapy recipients treated with dupilumab for the management of cutaneous immune-related adverse events. J Immunother Cancer. 2025;13(5):e010638. doi:10.1136/jitc-2024-010638
- Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569-581. doi:10.1016/j.annonc.2020.01.072
- Said JT, Talia J, Wei E, et al. Impact of biologic therapy on cancer outcomes in patients with immune checkpoint inhibitor-induced bullous pemphigoid. J Am Acad Dermatol. 2023;88(3):670-671. doi:10.1016/j.jaad.2022.06.1186
- Turner NC, Oliveira M, Howell SJ, et al. Capivasertib in hormone receptor-positive advanced breast cancer. N Engl J Med. 2023;388(22):2058-2070. doi:10.1056/NEJMoa2214131
- Tam S, Zhou G, Trombetta M, et al. Topical corticosteroids for the prevention of severe radiation dermatitis: a systematic review and meta-analysis. Support Care Cancer. 2023;31(7):382. doi:10.1007/s00520-023-07820-5
Articles in this issue
3 months ago
Cosmetic Trends of Dermatologic Interest in 20253 months ago
Identifying Breast Issues Beyond the SkinNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.