Long-Term Real-World Data Show Cutaneous Benefits of Belimumab
Key Takeaways
- Cutaneous manifestations in SLE are challenging, causing pain and psychosocial burden, necessitating effective long-term treatment options.
- Belimumab, targeting BLyS, shows promise in treating CLE, with significant reductions in skin disease activity over time.
Belimumab shows significant long-term benefits for cutaneous lupus erythematosus, improving skin symptoms and maintaining efficacy.
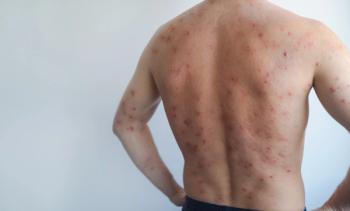
Cutaneous involvement is one of the most common and challenging aspects of systemic lupus erythematosus (SLE). Skin manifestations range from acute inflammatory eruptions to chronic scarring disease and non-specific lesions such as vasculitis. For many patients, these lesions are painful, disfiguring, and associated with a significant psychosocial burden. Despite standard systemic therapies, cutaneous disease may remain active, highlighting the need for effective long-term treatment options.1
Belimumab, a monoclonal antibody targeting B-lymphocyte stimulator (BLyS/BAFF), is approved for antibody-positive SLE and has demonstrated overall disease-modifying effects. While pivotal trials suggested improvement in mucocutaneous disease, skin outcomes were not assessed using validated dermatologic scoring systems. As a result, real-world data focusing specifically on cutaneous lupus erythematosus (CLE), particularly over extended treatment periods, have been limited.2
A recently published retrospective study from a German university center helps address this gap by examining the long-term effects of belimumab on cutaneous manifestations of SLE in routine clinical practice.3 The analysis included 29 adult patients with confirmed SLE and active skin disease, defined by a CLASI score of at least 2. All patients received belimumab for a minimum of 6 months, either subcutaneously or intravenously, and were followed for up to 5 years.
Cutaneous disease activity was assessed using the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) and the Revised CLASI (RCLASI), both validated tools commonly used in dermatology. Skin assessments were performed at baseline, at 3- and 6-month intervals during the first year, and annually thereafter.
Across the cohort, belimumab treatment was associated with a consistent and statistically significant reduction in skin disease activity. Mean CLASI scores decreased as early as 3 months after treatment initiation and continued to improve over time. At baseline, the mean CLASI score was 8.76, decreasing to approximately 5.6 at 3 months and to below 4 by 12 months. Improvements were largely maintained through longer follow-up, including assessments at 36, 48, and 60 months, although fewer patients were available for evaluation at later time points.
Clinical response rates reinforced these findings. A CLASI-20 response, defined as at least a 20% improvement in skin activity, was observed in most patients by 6 months and persisted throughout follow-up. Higher response thresholds were also achieved over time. Nearly half of patients reached a CLASI-50 response at several time points, and complete cutaneous remission (CLASI-100) was observed in a subset of patients, including one-third of evaluable patients at 5 years. Investigators noted these results suggest that belimumab’s cutaneous benefits may accumulate gradually with continued therapy.
The investigators also examined responses across different cutaneous lupus subtypes. Patients with acute cutaneous lupus, chronic discoid lupus, and non-specific lupus skin lesions showed early improvement. In contrast, patients with subacute cutaneous lupus did not demonstrate a statistically significant response at early time points. However, the small number of patients in each subgroup limits firm conclusions, and the authors note that these findings should be interpreted with caution.
Skin biopsies were taken from 4 patients at baseline and again from patients after 2 years of treatment, which were analyzed to explore changes in BLyS expression. Consistent with prior studies, lesional lupus skin showed increased BLyS expression compared with healthy controls. After prolonged belimumab therapy, BLyS staining in the skin was reduced, suggesting a local tissue effect of systemic BLyS inhibition. While preliminary, this observation raises interest in BLyS as a potential biomarker of cutaneous disease activity.
Belimumab was generally well tolerated throughout long-term treatment. The most commonly reported adverse events were mild to moderate infections, headache, and arthralgia, consistent with the known safety profile of the drug. No patients discontinued therapy due to adverse effects, and temporary treatment interruptions were uncommon.
Although limited by its retrospective design and small sample size, this study adds valuable long-term data to the evolving treatment landscape for cutaneous lupus. Larger prospective studies will be important to confirm these findings and to better define which patient subgroups are most likely to benefit from belimumab therapy.
References
- Stull C, Sprow G, Werth VP. Cutaneous involvement in systemic lupus erythematosus: A review for the rheumatologist. J Rheumatol. 2023;50(1):27-35. doi:10.3899/jrheum.220089
- Vilas-Boas A, Morais SA, Isenberg DA. Belimumab in systemic lupus erythematosus. RMD Open. 2015;1(1):e000011. Published 2015 Mar 3. doi:10.1136/rmdopen-2014-000011
- Schellin D, Rösing S, Zimmermann N, Leuchten N, Aringer M, and Günther C. Effect of belimumab on the cutaneous manifestations of patients with lupus erythematosus. Int J Dermatol. 2026. doi:10.1111/ijd.70251.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.