- Dermatology Times, October 2025 (Vol. 46. No. 10)
- Volume 46
- Issue 10
Unseen Delays: How Psoriasis Disrupts the Wound Healing Landscape
Key Takeaways
- Psoriasis is a systemic inflammatory disease that affects wound healing, complicating tissue repair and prolonging inflammation.
- Key cytokines such as IL-17, IL-23, and TNF-α disrupt wound healing stages, leading to chronic inflammation and impaired repair.
Psoriasis complicates wound healing due to systemic inflammation, affecting recovery and requiring tailored clinical approaches for optimal patient outcomes.
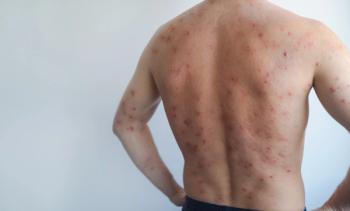
Psoriasis, often reduced to its visible hallmark of erythematous, scaly plaques, is increasingly recognized as a chronic, systemic inflammatory disease. The skin manifestations may bring patients to the clinic, but they tell only part of the story.
In recent decades, research has underscored the link between psoriasis and systemic comorbidities, including psoriatic arthritis, cardiovascular disease, and metabolic syndrome.1 Yet, one key domain remains relatively underrecognized: wound healing.
As dermatologists expand their procedural roles—whether performing skin biopsies, cryosurgery, aesthetic procedures, or excisions—recognizing the wound healing impairments in patients with psoriasis becomes imperative.
Inflammatory dysregulation in these patients not only alters immune responses but can also substantially delay or complicate tissue repair.2 This review explores how psoriasis reshapes the wound microenvironment, the consequences for healing, and the implications for clinical care.
A Systemic Inflammatory Disorder
The pathogenesis of psoriasis involves persistent activation of both innate and adaptive immune responses. Key cytokines such as IL-17, IL-23, and TNF-α drive the inflammatory cascade and maintain the hyperproliferative state of the epidermis.3
However, these same mediators also interfere with the tightly regulated stages of wound healing—hemostasis, inflammation, proliferation, and remodeling.4
In the context of psoriasis, the inflammatory phase of healing becomes prolonged and dysregulated. Cytokines central to psoriatic inflammation inhibit essential cellular functions in wound repair. For example, TNF-α impairs fibroblast migration and function, IL-17 alters angiogenesis, and IL-23 promotes a proinflammatory macrophage phenotype rather than a reparative one.5 These disruptions can cause wounds to remain in a chronic inflammatory state, failing to transition toward resolution.
What Goes Wrong in Wounds?
Multiple layers of the wound healing process are disrupted in psoriasis. Blood vessels may form inadequately, restricting oxygen and nutrient delivery. Fibroblasts—critical for collagen synthesis and extracellular matrix (ECM) production—lose functionality in inflamed environments.6 As a result, the ECM may break down prematurely or fail to form altogether.
This biochemical derailment explains why certain wounds in patients with psoriasis stall or fail to heal. Clinical signs of this phenomenon may be subtle: a clean surgical wound that unexpectedly dehisces, a punch biopsy site that remains open weeks later, or a wound edge that appears pale and fragile rather than vascular and granulating. Inflammatory infiltrates around these wounds may mimic infection, yet cultures remain negative.7
Furthermore, the Koebner phenomenon, in which trauma induces psoriatic lesions, complicates the picture further. New psoriasis plaques may develop at wound margins, blurring the line between healing tissue and disease flare.8 Even when healing is ultimately achieved, it may result in atrophic or cosmetically unsatisfactory scars.
The Role of Biologic Therapies
Biologic therapies targeting IL-17, IL-23, and TNF-α have revolutionized psoriasis care for patientss. In theory, by quelling systemic inflammation, biologics should enhance the wound healing process in patients with psoriasis. Indeed, patients who are well controlled on these agents often exhibit near-normal healing responses.
However, perioperative management of biologics remains a clinical gray zone. Discontinuing biologics prior to surgery may be recommended to lower infection risk, yet doing so risks triggering a psoriasis flare—which can itself derail healing.9
TNF inhibitors, in particular, have been associated with impaired wound repair in some settings, whereas IL-17 and IL-23 inhibitors may carry a more favorable risk profile.10
Therefore, individualized perioperative planning is essential for optimal outcomes. Coordinating care between proceduralists and the prescribing dermatologist can reduce risks. Providers should assess not only the visible activity of psoriasis but also consider subclinical inflammation when evaluating the healing capacity.
Recognizing Delayed Healing
Delayed wound healing in psoriasis is not rare—but it is often overlooked. In many cases, neither patient nor provider connects slow wound repair to psoriatic disease activity. The delays may be misattributed to infection or technical issues rather than to immune dysregulation.2
Even in well-controlled disease, low-grade systemic inflammation can impair angiogenesis, ECM remodeling, and fibroblast activity.11 This may lead to unnecessary antibiotic use, patient frustration, and delayed recovery.
By anticipating these delays in wound healing, clinicians can optimize care using gentler surgical techniques, adjunctive topical anti-inflammatories, and modified healing timelines.
Routine counseling for patients with psoriasis undergoing skin procedures should include discussion of possible delayed healing. Setting realistic expectations, arranging closer follow-up, and proactively managing inflammation can greatly improve outcomes.
Where Research Needs to Go Next
Despite growing awareness, wound healing impairment in psoriasis remains largely understudied. Most attention has focused on cardiovascular and joint complications, leaving this area relatively neglected.12 Several critical questions remain:
- Do cytokine profiles predict wound healing capacity?
- Do biologics improve or impede healing?
- What is the effect of disease control at the time of surgery?
A strong case exists for translational and clinical trials focusing specifically on procedural outcomes in psoriasis populations. Incorporating wound biomarkers (eg, TGF-β, VEGF, collagen turnover markers) into these studies may yield actionable insights.
Impaired Wound Healing in Psoriasis
While delayed wound healing in psoriasis is gaining attention, several underexplored areas merit further investigation:
- Wound microbiome: Disruption of the skin microbiome in patients with psoriasis may contribute to poor epithelialization or increased infection risk.13
- Metabolic comorbidities: Psoriasis commonly coexists with diabetes and obesity—conditions that independently impair wound repair.14
- Topical biologic and Janus kinase inhibitor use: Local delivery of anti-inflammatory agents may offer adjunctive benefits in postbiopsy or surgical wounds.15
- Long-term tissue integrity and scarring: Psoriatic inflammation may affect not only the speed of wound closure but also the quality of healed tissue.16
Incorporating these domains into future studies can help clarify the multifactorial barriers to optimal healing in this population.
Inflammation Beneath the Surface
Psoriasis alters not only how skin looks, but how it repairs. The chronic inflammation that defines the disease disrupts the very architecture of wound healing—compromising fibroblasts, microvasculature, and the extracellular matrix. These impairments are not always obvious, especially when disease is clinically quiescent.
For clinicians, especially dermatologic surgeons, acknowledging this hidden vulnerability can change how we approach care. It means choosing suture techniques that minimize tension, pausing to consider biologic timing, and staying alert to signs of wound compromise that are not infectious in nature.
As the therapeutic landscape for psoriasis continues to evolve, wound healing must be recognized as a critical, measurable outcome of care. By integrating this awareness into routine practice—and by expanding the evidence base—we can better align treatment strategies with the hidden, healing needs of our patients.
Gili Amid-Toby is a fourth-year medical student at St George’s University and a dermatology research fellow at the University of Miami.
References
Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):775.e1-775.e6. doi:10.1016/j.amjmed.2011.03.028
Young PM, Parsi KK, Schupp CW, Armstrong AW. Psoriasis and wound healing outcomes: a retrospective cohort study examining wound complications and antibiotic use. Dermatol Online J. 2017;23(11). doi:10.5070/D32311037238
Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227-255. doi:10.1146/annurev-immunol-032713-120225
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi:10.1126/scitranslmed.3009337
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957-970. doi:10.1056/NEJMra1505557
Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219-1222. doi:10.1172/JCI32169
Yeo QY, Kaliya-Perumal AK, Oh JY. Psoriasis complicating wound healing after minimally invasive lumbar spinal fusion. Indian Spine J. 2020;3(2):265–270. doi:10.4103/isj.isj_51_19
Weiss G, Shemer A, Trau H. The Koebner phenomenon: review and update. J Eur Acad Dermatol Venereol. 2002;16(3):241-248. doi:10.1046/j.1473-2165.2002.00406.x
Bakkour W, Purssell H, Chinoy H, Griffiths CE, Warren RB. The risk of post-operative complications in psoriasis and psoriatic arthritis patients on biologic therapy undergoing surgical procedures. J Eur Acad Dermatol Venereol. 2016;30(1):86–91. doi:10.1111/jdv.12997
Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):2427. doi:10.3390/ijms18112427
Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599-610. doi:10.1007/s12325-017-0478-y
Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377-390. doi:10.1016/j.jaad.2016.07.064
Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244-253. doi:10.1038/nrmicro2537
Gisondi P, Fostini AC, Fossà I, et al. Psoriasis and the metabolic syndrome. Clin Dermatol. 2018;36(1):21-24. doi:10.1016/j.clindermatol.2017.09.005
Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173(4):949–961. doi:10.1111/bjd.14018
Kasprowicz-Furmańczyk M, Narbutt J, Borzęcki A, Owczarczyk-Saczonek A. Does molecular scarring in psoriasis exist? a review of the literature. Postepy Dermatol Alergol. 2023;40(4):473–480. doi:10.5114/ada.2023.129322
Articles in this issue
3 months ago
Cosmetic Trends of Dermatologic Interest in 20253 months ago
Identifying Breast Issues Beyond the SkinNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.