
Studies offer positive outcomes for patients with conditions like hidradenitis suppurativa or psoriasis.

Studies offer positive outcomes for patients with conditions like hidradenitis suppurativa or psoriasis.

A retrospective cohort study published in the International Journal of American Academy of Dermatology investigated the possible association between atopic dermatitis and hidradenitis suppurativa.

In this week’s Pointers with Dr Portela, the 208SkinDoc watches several TikTok videos in which creators share their experience with hidradenitis suppurativa.

Ginette A. Okoye, MD, FAAD, provides several clinical pearls to help physicians find the best therapy for their individual hidradenitis suppurativa patients in her presentation at the Skin of Color Update 2021.

Many conditions are different in the pediatric population than in the adult population. How does hidradenitis suppurativa differ between the 2 groups?

Though investigators called for more research on the biologic, recent data showed patients treated with bimekizumab recorded improved skin pain when compared to placebo and adalimumab.

Novel biologics are moving into off-label indications to expand the dermatologic armamentarium for a broad range of inflammatory skin diseases.

Thanks to new drug approvals, medical management is replacing surgery as the first-line treatment for inflammation caused by hidradenitis suppurativa. However, surgery is often necessary to relieve pain related to tunnels.

Hidradenitis suppurativa has come to be associated with systemic inflammation as well as a growing list of comorbidities including acne conglobate.

Efforts are underway to identify new therapeutic methods that can offer improved outcomes for patients with hidradenitis suppurativa.

Clinical trials investigating the efficacy of biologics in treating this condition at Stage 2 and Stage 3 are growing in number and should be considered a front-line treatment option, says this expert.

Healthcare disparities exist in all fields of medicine, including dermatology. We seek to address these disparities in this article by outlining the differences in epidemiology, presentation, access and outcomes of five conditions in patients with skin of color.
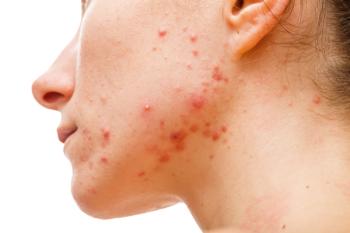
Patients with hidradenitis suppurativa have a high prevalence of acne vulgaris, according to the latest research.

With one approved biologic available and many more under investigation, the future of treatment for hidradenitis suppurativa looks very promising.

Adalimumab (Humira, AbbVie) enhances both health-related quality of life and work productivity in patients with moderate-to-severe hidradenitis suppurativa, according to the results of two phase 3 clinical trials.

Patients with hidradenitis suppurativa (HS) need wound care not only after surgery, to address HS scarring and tunneling from sinus tracts; they also need ongoing wound care for lesions that might be draining, says one dermatologist.

This month, we take a look at a treatment approach to hidradenitis suppurativa, the impact of atopic dermatitis on pediatric patients and a way to prevent mycosis fungoides progression.

Hidradenitis suppurativa can impact children and teens physically and emotionally. A dermatologist explains potential differences between pediatric and adult HS.

Expert dermatologist describes how to create the virtual multispecialty clinic to address the complex needs of hidradenitis suppurativa.

Nonablative lasers, which are essentially modified hair removal lasers, offer treatment benefits for HS patients with stage 1 or stage 2 disease, according to dermatologist.

TNF inhibitors adalimumab and infliximab have evidence-based efficacy for the treatment of hidradentitis suppurativa (HS). Anti-interleukin inhibitors ustekinumab and anakinra also being evaluated in small studies for treatment of HS. Antibiotics, hormones, retinoids, steroids and laser therapies directly targeting the lesions round out an effective treatment plan for HS patients.

Researchers treated a small cohort with two anti-inflammatory drugs and saw a significant improvement in clinical manifestations of disease.Further data showed the approach safely maintained results.

Evidence supports combined treatments to target the many factors tied to hidradenitis suppurativa.

Misconceptions about hidradenitis suppurativa (HS) prevent dermatologists and other clinicians from properly diagnosing and treating what one expert says is a “crippling disease.”

Many physicians miss diagnosing hidradenitis suppurativa upon first presentation. Recognizing key traits may help physicians diagnose and begin treatment of the disease earlier.