- Dermatology Times, February 2022 (Vol. 43. No. 2)
- Volume 43
- Issue 2
- Pages: 28
HS Steps Into Innovation Spotlight
Clinical trials investigate biologics, JAK inhibitors, and additional systemic treatments as first-line alternatives to or adjuvant therapies alongside surgery.
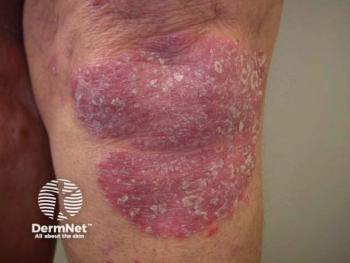
Hidradenitis suppurative (HS) could be the next beneficiary of the growing body of research on the cytokine pathways underlying inflammatory skin disease, according to Mirza A. Alikhan, dermatologist at Sutter Health in Sacramento, California.
Speaking at Maui Derm for Dermatologists 2022, held January 24 to 28, 2022, in Hawaii, Alikhan previewed some of the off-label and pipeline drugs taking aim at target that would inhibit the effects of inflammation-causing fibroblasts involved in the pathogenesis of this disease. He also highlighted tips for treating challenging cases in patients with moderate to sever HS.1
“Until now, dermatologists had to ‘recycle’ some of the breakthrough drugs that recently advanced treatment of other inflammatory skin diseases for their patients with HS,” he told Dermatology Times® in a follow-up interview. “Everybody was so obsessed with, for example, psoriasis. In just a very short amount of time, there were many new options for moderate to severe psoriasis. That’s kind of happening with atopic dermatitis [AD]. I think HS is the next big frontier for a lot of this type of development.”
In fact, more than 100 clinical trials have been announced or are under way for various biologics, Janus kinase (JAK) inhibitors, and other systemic drugs developed to combat the causes of this inflammatory condition.2
At the top of his watch list: biologic therapies, which are being tested on more precise molecular targets or combined pathways. “Today, adalimumab [Humira; AbbVie] is the only FDA approved treatment for moderate to severe HS,” said Alikhan, who was the lead author on the 2019 North American clinical guidelines for HS.3
“We have seen how big a game changer it has been. Having more systemic options and more data would give physicians additional tools to develop individualized treatment plans for this very complex disease.”
Alikhan is particularly interested in clinical trial results for IL-17 and IL-17a inhibitors. He pointed out “some promising data” for medications in the FDA pipeline, including 2 phase 3 studies of the possible new indication for secukinumab (Cosentyx; Novartis) and a phase 2 study for bimekizumab (Bimzelx; UCB). An investi- gational IL-17A and IL-17F inhibitor for treating moderate to severe plaque psoriasis, bimekizumab is being investigated as an option for HS.
Secukinumab’s phase 3 Sunrise and Sunshine (NCT03713632, and NCT03713619) trials, which met their primary end points for skin clearance vs placebo, are in extension studies continuing to 52 weeks, with expected comple- tion in the second quarter of 2022.4 Results of a phase 2 double-blind, placebo-controlled, randomized clinical trial investigating bimekizum- ab’s efficacy and safety in moderate to severe HS (NCT03248531) showed that the drug demonstrated a higher HS clinical response (HiSCR) rate compared with placebo (57.3% vs 26.1%) at week 12, as well as greater clinical improvement.5
IL-1 is also getting attention, Alikhan said. Results of a phase 2 open-label, recombinant clinical trial of bermekimab (JNJ-77474462; Janssen Biotech) showed that 60% of patients treated with this human monoclonal antibody achieved HiSCR vs 10% in the placebo group. According to the authors, bermekimab “neutralizes IL-1α by binding this cytokine with high affinity and thereby acts as a blocker of IL-1α activity.” The small study (n = 20) involved patients who had refractory HS or were 6 ineligible for adalimumab, the authors wrote.
Complement pathways offer another interesting avenue of investigation for treating HS, Alikhan said. He pointed to research results showing that the complement pathway through C3 and C5 was found to produce inflammation, opsonization, and bacterial lysis, according to the authors. C3a and C5a “are strong neutrophil activators and recruiters,” they wrote, which suggests that complement inhibition “might be a line of action in HS treatment.” The authors also noted that patients with HS had high blood concentration levels of C5 compared with healthy subjects and that “reduction of nodules and draining dermal tunnels was observed following C5a inhibition.”7
JAK inhibitors also may be on the horizon for the HS armamentarium, Alikhan said. Incyte has a first-quarter 2022 start date for an exploratory trial (NCT04414514) of its topical JAK, ruxolitinib 1.5% cream (Opzelura), to treat early-stage HS.6 Opzelura gained FDA approval as an AD treatment last September but was required to carry a black box warning about the possibility of adverse effects including blood clots, cancer, and heart-related events such as heart attack and stroke.8 Estimated completion date for that trial is January 2024.
Does this free-flowing pipeline mean surgery will play only a minor role in HS? “When I was a resident over a decade ago, the conventional wisdom was that there was no point in treating HS medically because it is a surgical disease,” Alikhan said. “Then that shifted to the thinking that HS is a disorder of the immune system [and] that medication should be the first- line therapy. What I’ve seen in my practice is that both surgery and medications can work, and sometimes they work better together.”
In his view, surgery is a good choice for patients who have drainage in areas of the body that are hard for medications to reach; mayhave persistent disease; and are larger, with many lesions and tunnels. “I’ve had good outcomes pairing surgery with drugs such as adalimumab. Wide excision or deroofing can remove pesky, persistent pockets or disease-damaged tissue, but you still need medication to reduce inflammation,” Alikhan said.
Like the range of drug choices, surgery could be bracing for new approaches, such as use of preoperative ultrasoundography. Results of a recent study showed that ultrasoundography improved surgical margin delineation and reduced recurrence.8
“It’s not part of a regular practice yet, but physicians are discussing how this would fit into their practice,” Alikhan said. “I’ve seen so many ways where they use the ultrasound results as a way to monitor disease activity. It’s almost like the overlap of dermatology and radiology.”
All this underscores the fact that, although HS management is still complex, there are many more options than ever, and the pipeline will likely expand rapidly.
“Patients sometimes feel abandoned if the doctor tries a couple of things and then gives up. There are always different things we can try until [the paitnet and I] find something that works,” Alikhan said.
Disclosures
Alikhan reported no relevant disclosures.
References
1. Desai S, Rosen T, Alikhan, A, Treat J. Challenging cases. Presented at: Maui Derm for Dermatologists 2022; January 24-28, 2022; Maui, HI.
2. Hidradenitis suppurativa. ClinicalTrials.gov. Accessed January 17, 2022. https:// clinicaltrials.gov/ct2/results?cond=Hidradenitis+Suppurativa&recrs=b&recrs= a&recrs=f&recrs=d&recrs=e&age_v=&gndr=&type=&rslt=&Search=Apply
3. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guide- lines for hidradenitis suppurativa: a publication from the United States and Cana- dian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81(1):76-90. doi:10.1016/j.jaad.2019.02.067
4. Novartis R&D day spotlights attractive growth profile, underpinned by strong in-market brands, 20 potential high value pipeline assets, and technology plat- forms. News release. Novartis. December 2, 2021. Accessed January 15, 2022. https://www.novartis.com/node/83456/printable/
5. Jemec GB. Clinical practice. hidradenitis suppurativa. N Engl J Med. 2012;366(2):158-164. doi:10.1056/NEJMcp1014163
6. Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, Puig L. New perspec- tives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021. Published online November 23, 2021. Accessed January 18, 2022. doi:10.1177/20406223211055920
7. Del Duca E, Morelli P, Bennardo L, Di Raimondo C, Nisticò SP. Cytokine pathways and investigational target therapies in hidradenitis suppurativa. Int J Mol Sci. 2020;21(22):8436. doi:10.3390/ijms21228436
8. Cuenca-Barrales C, Salvador-Rodríguez L, Arias-Santiago S, Molina-Leyva A. Pre-operative ultrasound planning in the surgical management of patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34(10):2362- 2367. doi:10.1111/jdv.16435
Articles in this issue
almost 4 years ago
Experts Tackle Skin-Lightening Controversyalmost 4 years ago
Essential Treatment Tips for Textured Hairalmost 4 years ago
Experts Weigh in on Best Body-Focused Skin Carealmost 4 years ago
How to Explain JAK Inhibitor Warnings to Patientsalmost 4 years ago
Get to the Roots of Pediatric Acnealmost 4 years ago
Legal and Tax Options for a Practice’s Structurealmost 4 years ago
Cosmetic Conundrums & Cleansing Complexitiesalmost 4 years ago
OSHA, the Needle, and the Fineabout 4 years ago
What You Need to Know About Mentoring Doctorsabout 4 years ago
Trending Now: Pipeline Possibilities for Pediatric Skin ConditionsNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.