
Hiring investigators of color, educating and building trust with potential participants, and addressing funding can make clinical trials more diverse.

Hiring investigators of color, educating and building trust with potential participants, and addressing funding can make clinical trials more diverse.
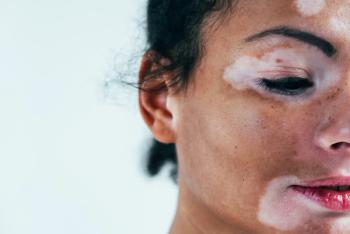
Frank discussions about managing the disorder can boost patients’ self-esteem and quality of life.

Pearl Grimes, MD, FAAD, and director of the Grimes Center of Medical and Aesthetic Dermatology and the Vitiligo & Pigmentation Institute of Southern California in Los Angeles, explains why the FDA approval of ruxolitinib cream 1.5% is monumental in the treatment of vitiligo.

Ruxolitinib (Opzelura) cream is the first and only FDA-approved treatment for patients with vitiligo.

At the SPD 2022 47th annual meeting, investigators share data on the treatment of ruxolitinib for this chronic autoimmune disease.

At the SPD 2022 47th Annual Meeting, researchers delve into the safety and practicality of using narrow-band UVB phototherapy on children with vitiligo.

An extraordinary display of posters at this year's Society for Pediatric Dermatology annual conference.

Ruxolitinib cream 1.5% may soon be the first FDA-approved treatment for repigmentation of vitiligo. Experts share what dermatologists need to know regarding efficacy, safety, and cost of this topical drug.

In this drug pipeline video, Pearl Grimes, MD, FAAD, and Seemal R. Desai, MD, FAAD discuss what's currently happening in the vitiligo pipeline including why current treatments are lacking, what's coming soon, and what it may cost.

Vitiligo is challenging to treat; fortunately, continued research has led to improvements and refinements in options, giving much-needed hope to patients with this skin condition.

VYNE Therapeutics announced positive preclinical data of a lead BET inhibitor reducing melanocyte loss and key inflammatory biomarkers from an ex vivo skin model of vitiligo.

Long underserved by a narrow armamentarium, patients with pigmentary disorders could soon see a new wave of oral and topical solutions to treat even the most challenging cases.

This episode of The Cutaneous Connection podcast, sponsored by Incyte, explores the world of vitiligo. From symptoms to current treatment dissatisfaction and the latest research, Pearl Grimes, MD, FAAD, explains where we are now with this pigmentary disorder and where we are headed.

Ruxolitinib cream (Opzelura; Incyte) receives priority review of its supplemental New Drug Application as a treatment for patients with vitiligo.
Looking toward the future management of vitiligo, expert dermatologists close out their discussion on JAK inhibition and other novel therapy.

Shared insight from dermatologists on novel topical JAK inhibitor therapy being investigated as an option for patients with vitiligo.

A recent study examined the impact of virtual group visits to obtain community and education for pediatric vitiligo and alopecia areata patients and caregivers.

A brief review of the treatment armamentarium for vitiligo, as well as the prevalent unmet needs in that setting.

A recent JAAD study evaluated the correlation between F-VASI and T-VASI using patient- and physician-reported measures of clinical improvement, PaGIC-V and PhGVA.

Seemal Desai, MD, FAAD, and William Damsky, MD, PhD, discuss methods for managing a patient’s motivation and expectations when on therapy for vitiligo.

In this video interview, James Q Del Rosso, DO, tackles the clinical impact of JAK Inhibitors and how to deal with patient concern.

Expert perspectives on the diagnosis of vitiligo and considerations for improving provider awareness in dermatologist and community settings.

A comprehensive breakdown of the different classifications of vitiligo and how these may present clinically.

In this exclusive video interview, James Q Del Rosso, DO, gets into the details of JAK inhibitors, explaining how they work and cutting through the headlines around the treatments.

Shared insight on the psychological and social impact of vitiligo, along with practical advice on how to communicate with and support patients.