
The study found that higher doses of BTX “significantly” improved facial erythema and patient quality of life compared to lower doses.

The study found that higher doses of BTX “significantly” improved facial erythema and patient quality of life compared to lower doses.
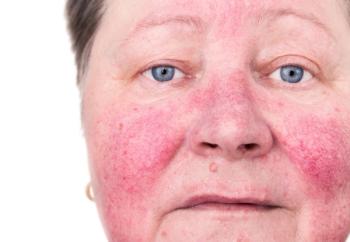
Mendelian randomization suggested a potential causal relationship between the 2 variables.

The study found 277 differentially expressed genes shared by RA and rosacea, opening up opportunities for treatment research.
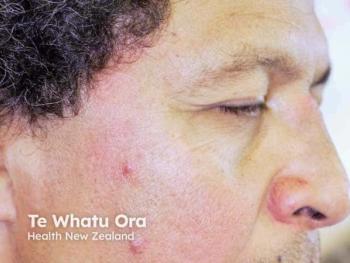
Recent research evaluated 6 patients with rosacea treated with JAK1 inhibitors upadacitinib and abrocitinib.

A review of 43 studies found that systemic treatments generally provide greater reductions in rosacea ILs compared to topical therapies.

A recent study reviewed the current evidence on the impact of key vitamins and minerals on rosacea and provided clinical recommendations.
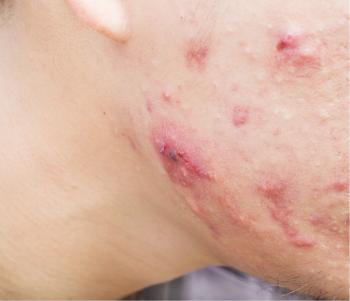
Some patients may be looking for alternatives to BPO, questioning its safety.

A recent review found the antifibrinolytic drug performed well in several clinical studies, none of which reported severe adverse events.

Researchers found evidence from numerous studies claiming LDI could improve rosacea symptoms and decrease flare-ups.

Scores for erythema, dryness, quality of life, and aesthetic improvement were all markedly improved in patients using tranexamic acid versus traditional therapy alone.

Earlier this week, we shared our fourth and final Rosacea Awareness Month quiz. Review the answers and your responses below.

Review Dermatology Times' coverage of Rosacea Awareness Month.

Click here to read more and answer our quiz questions in recognition of Rosacea Awareness Month.

Earlier this week, we shared our third Rosacea Awareness Month quiz. Review the answers and your responses below.

Chinese clinical researchers recently presented a unique case of JLIS manifesting as erythematous papules and infiltrative plaques on the nose and upper jaw, resembling rosacea.

Click here to read more and answer our quiz questions in recognition of Rosacea Awareness Month.

Earlier this week, we shared our second Rosacea Awareness Month quiz. Review the answers and your responses below.

Researchers compared a variable-sequenced, large-spot 532 nm KTP laser to the 595 nm pulsed-dye laser.

In recognition of Rosacea Awareness Month, Dermatology Times is reviewing research and strides in rosacea treatment over the last decade.

Click here to read more and answer our quiz questions in recognition of Rosacea Awareness Month.
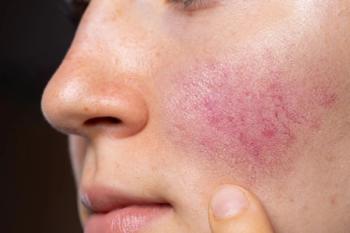
Earlier this week, we shared our first Rosacea Awareness Month quiz. Review the answers and your responses below.

This Rosacea Awareness Month, take a look at previous coverage from 2024.

This Rosacea Awareness Month, we have compiled a list of resources for clinicians to share with their rosacea patients.

Click here to read more and answer our quiz questions in recognition of Rosacea Awareness Month.

One study uncovered high levels of CGRP in patients with rosacea, with levels not affected by age, sex, BMI, and other factors.