- Dermatology Times, November 2025 (Vol. 46. No. 11)
- Volume 46
- Issue 11
Defining Dermatology’s Year-End Surge
Key Takeaways
- Recent FDA approvals include treatments for chronic hand eczema, atopic dermatitis, plaque psoriasis, psoriatic arthritis, and CSU, highlighting significant advancements in dermatology.
- Remibrutinib's approval for CSU has prompted discussions on enhancing patient care through improved collaboration between dermatologists and allergists.
Explore the latest FDA approvals in dermatology, including treatments for eczema and psoriasis, and gain insights on chronic spontaneous urticaria.
As 2025 approaches its final months, Dermatology Times is reflecting on the numerous FDA approvals and updates that impacted the dermatology community in the third and fourth quarters of the year. To name a few, LEO Pharma’s delgocitinib cream (Anzupgo) became available in the US for the treatment of adults with moderate to severe chronic hand eczema; Incyte’s ruxolitinib cream (Opzelura) received an expanded indication for pediatric patients aged 2 to 11 years with mild to moderate atopic dermatitis; the FDA approved Johnson & Johnson’s guselkumab (Tremfya) for the treatment of pediatric patients aged 6 years and older with moderate to severe plaque psoriasis or active psoriatic arthritis; and Novartis’ remibrutinib (Rhapsido) received FDA approval for the treatment of adults with chronic spontaneous urticaria (CSU).
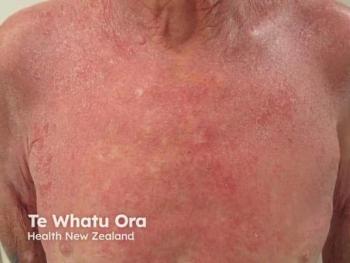
With remibrutinib being the FDA’s latest approval, clinicians have been discussing in-depth the novel Bruton tyrosine kinase inhibitor’s entry into the CSU space. To dive further into CSU and how to best support patients, Naiem Issa, MD, PhD; and David M. Lang, MD, detail how dermatologists and allergists can work together in this month’s cover collection on CSU. As Issa and Lang note, “The journey toward CSU diagnosis and relief is often fraught with frustration due to lack of consistent adherence with diagnostic and treatment guidelines, and a relative lack of collaboration in decision-making between providers and patients.”
In this month’s cover feature on CSU, don’t miss exclusive insights from the key approval from Christopher Bunick, MD, PhD; and Walter Liszewski, MD.
Other notable features in this month’s issue include a look at Oruka Therapeutics’ ORKA-001 for the treatment of psoriasis and insights from Andrew Blauvelt, MD, MBA; a Q&A with Jorge Garcia-Zuazaga, MD, MBA, of Apex Skin on clinical trial access in private practice models; and a conversation with Emma Guttman-Yassky, MD, PhD, on ritlecitinib (Litfulo; Pfizer) for scarring alopecia.
Stay tuned to Dermatology Times through the end of the year for updates from the 2025 Fall Clinical Dermatology Conference, the 2025 Society of Dermatology Physician Assistants Annual Fall Dermatology Conference, and the Elevate-Derm Fall Conference.
As always, Dermatology Times delivers the most up-to-date clinical content, from innovative studies and FDA approvals to exclusive interviews and expert perspectives. Stay informed with the latest data—subscribe to Dermatology Times’ e-newsletters; follow us on Facebook, LinkedIn, and X.
Mike Hennessy Jr
Chairman and CEO
MJH Life Sciences
Articles in this issue
about 2 months ago
Dermatology Times November 2025 Print Recapabout 2 months ago
Q4 Investment Planning: What to Do Now to Reduce What You Owe in 20262 months ago
Skin Aging and Cellular Senescence2 months ago
Clinicians Debate True Sensitive Skin Care3 months ago
ORKA-001 Advances Toward Yearly DosingNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.