- Dermatology Times, November 2025 (Vol. 46. No. 11)
- Volume 46
- Issue 11
Case-Based Strategies for Optimizing AD Care
Key Takeaways
- Ruxolitinib cream demonstrated rapid itch relief and skin clearance in moderate AD, as evidenced by phase 3 trial results.
- Tapinarof cream was chosen for a teen with refractory AD, showing promise in achieving clear skin in the ADORING 3 trial.
During a Case-Based Roundtable event, Naiem Issa, MD, PhD, guided colleagues through 3 complex atopic dermatitis cases, highlighting how modern topicals can deliver rapid and durable control across age groups.
“When I present data, I say, ‘You want to give this cream a chance. This cream alone could stave off the need for using a biologic.’ That usually resonates with some of my patients,” said Naiem Issa, MD, PhD, at a recent
Issa, director of clinical trials and research at Forefront Dermatology, clinical assistant professor at the George Washington University School of Medicine and Health Sciences in Washington, DC, and assistant professor at the University of Miami Leonard M. Miller School of Medicine in Miami, Florida, led colleagues through a discussion of 3 complex atopic dermatitis (AD) cases to discuss which available therapeutics may be best suited for each patient based on age, disease severity, and response to previous topicals.
Case 1
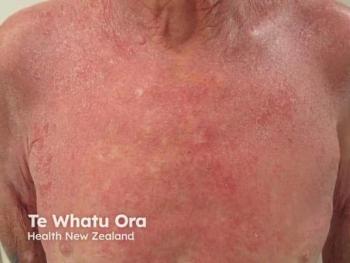
A 48-year-old man with AD since childhood presented with eczematous lesions involving approximately 10% of his body surface area (BSA) and severe pruritus affecting sleep and work performance. His disease was historically well controlled with topical therapies but has progressively worsened over the past year following occupational changes. Previous use of triamcinolone acetonide 0.1% provided moderate relief during flares; however, he now experiences more frequent and persistent exacerbations. A recent switch to topical tacrolimus failed to achieve symptom improvement, indicating inadequate disease control with topical agents and the potential need for escalation to systemic or biologic therapy.
As one of the attendees noted, BSA severity is one of the leading metrics used to quantify mild to moderate AD.
Issa added, “Ten percent BSA, according to the protocols, is what defines a moderate disease category, and 30% is supposed to define severe categories.”
After discussing treatment options with the patient, the dermatologist prescribed ruxolitinib 1.5% cream (Opzelura; Incyte), applied twice daily. The patient quickly experienced resolution of his itch, and his skin cleared with continued use.
During the discussion, Issa reviewed ruxolitinib’s rapid itch clearance data, highlighting results from the phase 3 TRuE-AD1 (
“Itch is the No. 1 most burdensome symptom in [AD]. That has been well studied and well validated in numerous patient-reported outcome studies,” Issa said.
He also reviewed 4-week data demonstrating that a greater percentage of patients treated with ruxolitinib cream 1.5% achieved Investigator’s Global Assessment Treatment Success (IGA-TS), Eczema Area and Severity Index (EASI) 75, EASI 90, and itch NRS 4 compared with topical triamcinolone 0.1%.2
Case 2
A 17-year-old teen with AD diagnosed at age 2 years and a higher Fitzpatrick skin type presented with persistent disease predominantly affecting the neck and hands. His symptoms worsened during football season, and topical therapies no longer provided adequate control. Previous treatments include various topical corticosteroids with rebound flares after discontinuation, topical tacrolimus with moderate but temporary improvement, and crisaborole, which was discontinued due to burning upon application. Despite these interventions, the patient continued to experience frequent flare-ups and inadequate disease management, suggesting refractory AD that may warrant consideration of systemic or biologic therapy options.
Given persistent disease activity despite multiple topical therapies, systemic treatment with dupilumab (Dupixent; Sanofi and Regeneron) was recommended. However, the patient expressed reluctance to begin injectable therapy and preferred to pursue another topical option first. Consequently, treatment with tapinarof cream (Vtama; Organon), applied once daily, was initiated as the next step in management to assess potential improvement before escalation to systemic therapy.
Regarding patients with skin of color with AD, one attendee added, “Some patients with darker skin types and [AD] will say they are most worried about getting rid of their dark spots. I have to mention that to get rid of their dark spots, we have to get rid of the itch and the eczema first. I like to ask my patients what their No. 1 goal is.”
Issa reviewed data from the ADORING 3 open-label extension trial, where patients entered the ADORING 3 study (48 weeks) from previous tapinarof trials or as direct enrollees. Patients entering with a Validated Investigator Global Assessment scale for Atopic Dermatitis (vIGA-AD) greater than or equal to 1 received tapinarof until a vIGA-AD of 0 was achieved. Patients entering with vIGA-AD of 0 discontinued tapinarof and were assessed for maintenance of clear/almost clear skin off-treatment. Overall, 51.9% of patients using tapinarof cream achieved vIGA-AD 0 (complete clearance) and 81.6% of patients achieved vIGA-AD 0 or 1 (clear or almost clear).3
“We have 3 excellent nonsteroidal options since topical calcineurin inhibitors,” Issa said.
Case 3
An 8-year-old girl with AD diagnosed at age 3 years presented with worsening symptoms primarily affecting the flexural folds of the elbows and knees. Her parents report persistent pruritus that disrupts sleep and daily comfort. Previous treatments include intermittent topical corticosteroids with limited efficacy, topical calcineurin inhibitors providing only partial improvement, and oral antihistamines with minimal itch relief. Despite these interventions, disease control remains inadequate, suggesting the need to reassess her management plan and consider alternative or advanced therapies to address ongoing inflammation and pruritus.
With this case, Issa did point out boxed warnings with topical calcineurin inhibitors and noted that there is no increased incidence for infants, children, or adults for any malignancies.
Based on the patient’s age, Issa discussed with attendees data from the TRuE-AD3 trial (
References
- Blauvelt A, Szepietowski JC, Papp K, et al. Itch-free state in patients with atopic dermatitis treated with ruxolitinib cream: a pooled analysis from two randomized phase 3 studies. J Am Acad Dermatol. 2023;88(3):651-653. doi:10.1016/j.jaad.2022.09.010
- Kircik L, Sturm D, Kallender H, Xue Z, Nasir A. Ruxolitinib cream versus triamcinolone cream in adults with mild to moderate atopic dermatitis. J Drugs Dermatol. 2025;24(10):1036-1039. doi:10.36849/JDD.8920
- Bissonnette R, Stein Gold L, Kircik L, et al. Skin clearance, duration of treatment-free interval, and safety of tapinarof cream 1% once daily: results from ADORING 3, a 48-week phase 3 open-label extension trial in adults and children down to 2 years of age with atopic dermatitis. J Am Acad Dermatol. 2025;93(3):707-714. doi:10.1016/j.jaad.2025.05.1391
- Soong W, Zaenglein A, Tollefson M, et al. Long-term safety and disease control of ruxolitinib cream in children aged 2 to 6 and 7 to 11 years: results from the TRuE-AD3 study. J Allergy Clin Immunol. doi:10.1016/j.jaci.2024.12.599
Articles in this issue
about 2 months ago
Dermatology Times November 2025 Print Recapabout 2 months ago
Q4 Investment Planning: What to Do Now to Reduce What You Owe in 20262 months ago
Skin Aging and Cellular Senescence2 months ago
Clinicians Debate True Sensitive Skin Care3 months ago
ORKA-001 Advances Toward Yearly DosingNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.