
It has been 20 years since the FDA approved a new sunscreen filter. However, a recent study evaluating curcumin could change that.

It has been 20 years since the FDA approved a new sunscreen filter. However, a recent study evaluating curcumin could change that.
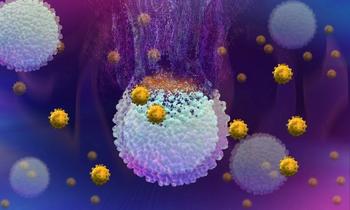
There are an increasing number of novel immunotherapies available for patients with skin cancer, however, their numerous adverse events must be addressed.

Curcumin has a long history as an effective treatment for everything from chronic pain to skin infections. Nanoparticle encapsulation shows promise in meeting that challenge and opening the way for a safe, effective adjuvant to sunscreen.

The FDA has granted priority review to Merck’s application for pembrolizumab, a treatment for melanoma.

Researchers from UT Southwestern Medical Center have investigated a new way to use AI to determine the metastatic potential of melanoma.

The FDA has granted priority review to a supplemental biologics license application for pembrolizumab for the use as an adjuvant treatment in adult and pediatric patients with stage IIB or IIC melanoma following complete resection.

In this episode, Alberto Pappo, MD, director, St. Jude Solid Tumor Division, discusses a registry he helped create aimed at helping physicians better understand pediatric melanoma called Molecular Analysis of Childhood MELanocytic Tumors (MACMEL).

In this video interview, David Light, CEO of Valisure, shares his thoughts on the recent sunscreen recall from Johnson & Johnson due to benzene contamination and reveals how the FDA and sun protection companies can make sure this never happens again.

Immunotherapy agent nemvaleukin alfa was granted an FDA fast track designation for the treatment of patient’s mucosal melanoma for previously undergone treatment with an anti-PD-L1 therapy.
In this video interview, Alberto Pappo, MD, director, St. Jude Solid Tumor Division, discusses a registry he helped create aimed at helping physicians better understand pediatric melanoma called Molecular Analysis of Childhood MELanocytic Tumors (MACMEL).

In this exclusive video interview, Christopher Bunick, MD, PhD, gives Dermatology Times an update on benzene contamination being detected in various sunscreens and after-sun care products, as well as a recent recall by Johnson & Johnson.
The FDA has granted an orphan drug designation to alrizomadlin as a potential therapeutic option for patients with stage IIB-IV melanoma.

Christopher Bunick, MD, PhD, provides an update on benzene contamination in sunscreen, including a recent recall from Johnson & Johnson.

St. Jude Children’s Research Hospital researchers have created a registry to better understand pediatric melanoma.

The combination of belapectin plus pembrolizumab has demonstrated early potential for disease control with acceptable tolerability in patients with metastatic melanoma.

OncoSec Medical Inc. and Merck have entered into a supply and clinical trial agreement for pembrolizumab (Keytruda) and tavokinogene telseplasmid (Tavo) for the potential treatment of late-stage metastatic melanoma.

DermTech offers a new way to detect skin cancer without patients having to go under the knife.
Lenvatinib and pembrolizumab continued to yield meaningful and durable responses in patients with advanced melanoma who had progressed on previous PD-L1 inhibitor treatment.

New regulations, latest absorption data, and updates on environmental and health impacts are shaping the world of photoprotection.

Christopher Bunick, MD, PhD, associate professor of dermatology, Yale University and Dermatology Times® editorial advisory board member, weighs in on a recent report revealing certain batches of various sunscreen and after-sun care products were contaminated with benzene, a carcinogen.

This episode will highlight some breaking news coming out of the sun protection world with a report by Valisure, which found in a recent test that 78 different sunscreen and after-sun care products were contaminated with benzene, a potential carcinogen.

David Light, founder and CEO of Valisure, the independent lab that discovered the presence of benzene in multiple brands and batches of sunscreen, sits down with Dermatology Times to discuss the reports' findings and the impact this news will have on the sun protection industry.

A recent test found various brands and batches of sunscreen and after-sun care products contained benzene, a potential carcinogen.

Content-aware image restoration (CARE) technology demonstrated improvement in examining cellular details of skin.

A study published in JAMA Dermatology investigated how consumers look at sunscreen active ingredients in response to an FDA proposed rule.