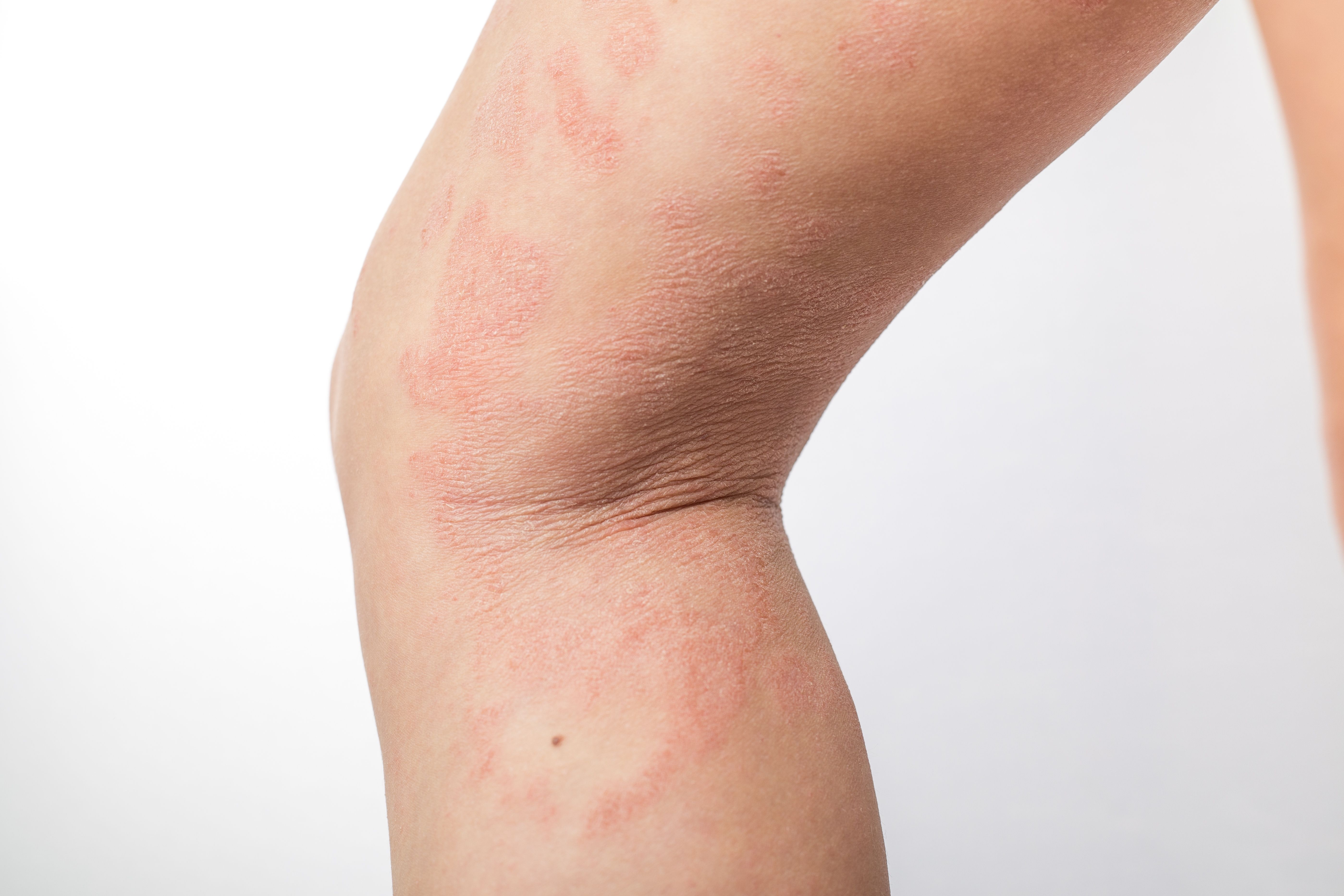
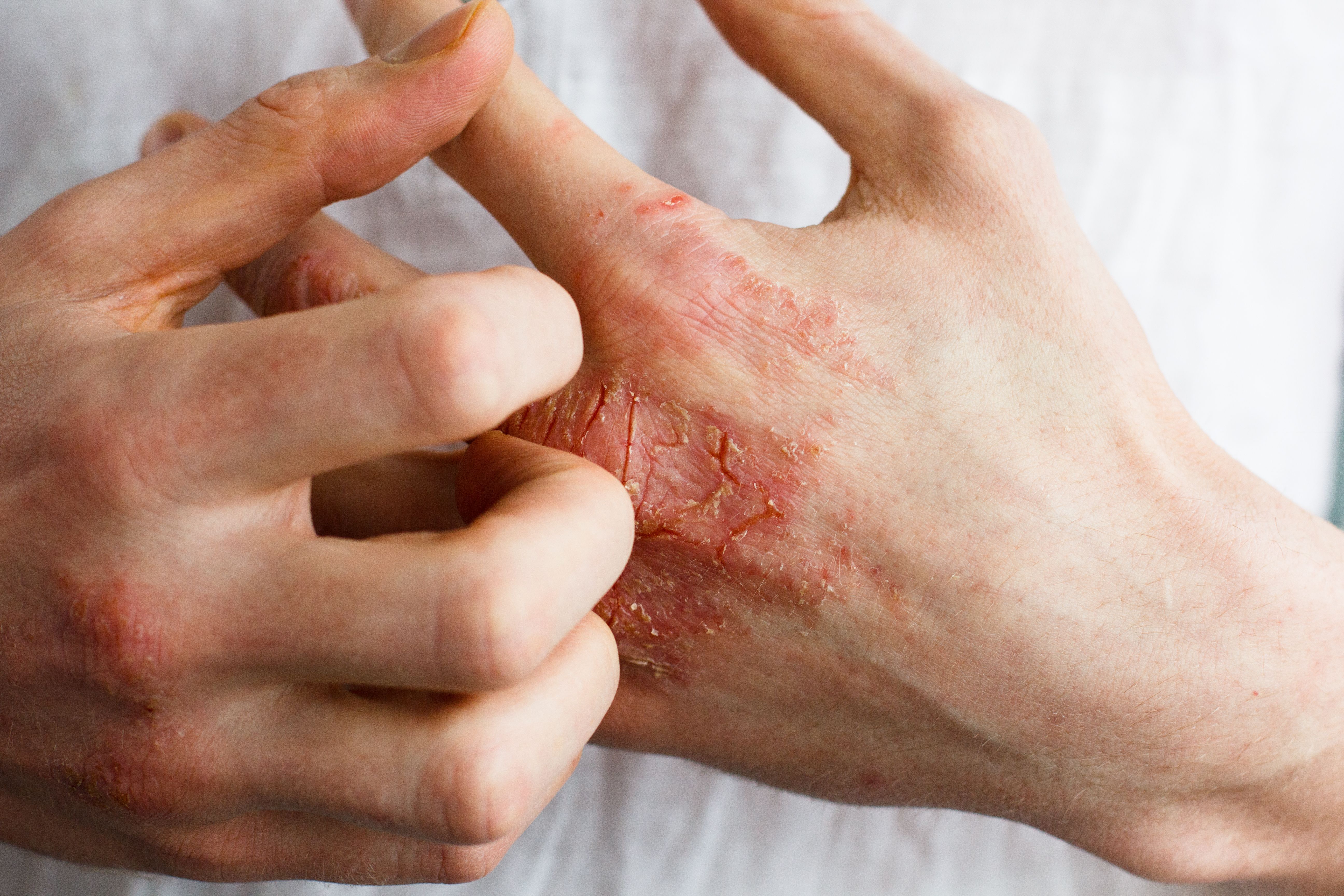
Atopic Dermatitis
Latest News
Latest Videos

CME Content
More News

Patients with mild to moderate AD have a new topical treatment option, but physicians will need to prepare for some focused conversations about safety.

At Maui Derm NP+PA Fall 2021, Vikash S. Oza, MD, and Matthew J. Zirwas, MD, discuss the current Rx and Dx of atopic dermatitis in children.

New therapies raise patient expectations for fast efficacy, lower costs, and fewer adverse effects.

Investigators added that the meaning of each laboratory marker is dependent on the drug that is being used for treatment.

NEA’s Eczema Expo highlights new drugs, steroid withdrawal, alternative therapies.

Incyte announced the FDA has approved its topical selective Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib (Opzelura) for the treatment of mild to moderate atopic dermatitis.

Verrica Pharmaceuticals has received a complete response letter from the FDA identifying deficiencies at a manufacturing facility for VP-102.

Derm-Biome’s topical compounds have positive results in preclinical trial.
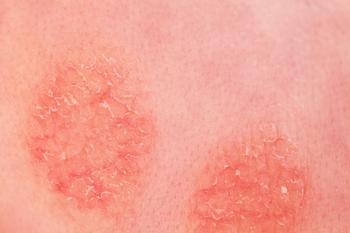
Dermavant has announced it has dosed the first patient in its phase 3 trial evaluating tapinarof cream for the treatment of moderate to severe atopic dermatitis.

A recent phase 3 trial investigated the safety of dupilumab as a treatment for severe atopic dermatitis in pediatric patients ages 6 to 11 years.
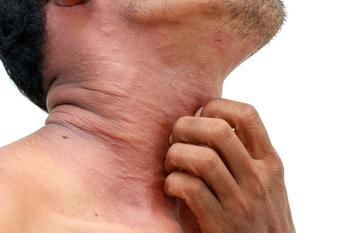
A new study sheds light on some of the atopic disease risks that are associated with early childhood and continue into midlife.

Mark Lebwohl, MD, takes a deep dive in the recent delay in approval for multiple JAK inhibitor drugs for treatment of inflammatory skin conditions such as psoriasis and atopic dermatitis. He also discusses the impact of the delay, as well as the benefits of JAK inhibitors for inflammatory skin diseases, and an outlook for the future of this drug class.

The FDA is now requiring black box warning for certain JAK inhibitors used to treat arthritis. This news comes amid delay in FDA approval for multiple JAK inhibitors for the treatment of inflammatory skin conditions.

Pfizer’s JADE DARE phase 3 trial met its coprimary and key secondary efficacy endpoints.

Regeneron Pharmaceuticals, Inc. and Sanofi announced safety and efficacy results from the LIBERTY AD trial investigating dupilumab in pediatric patients ages 6 months to 5 years with atopic dermatitis.

Upadacitinib has been approved by the European Commision and becomes the first Janus kinase (JAK) inhibitor approved in the European Union for the treatment of moderate to severe atopic dermatitis in adult and pediatric patients.

In this video interview, Marc Serota, MD, discusses his research regarding type 2 inflammation, the future of treating it as 1 condition or 2, and why physicians should educate their patients about this type of inflammation.
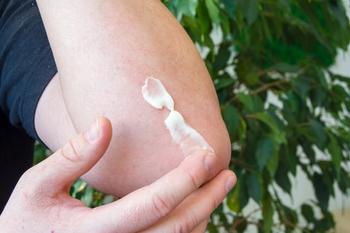
A phase 3 trial studied the effectiveness of oral abrocitinib along with topical therapy for moderate-to-severe atopic dermatitis in teens.

Eli Lilly announced that lebrikizumab has met all primary and key secondary endpoints at week 16 in their 2 phase 3 trials.

A study published in the Journal of the American Academy of Dermatology examined the potential relationship between air pollution and the incidence of atopic dermatitis.
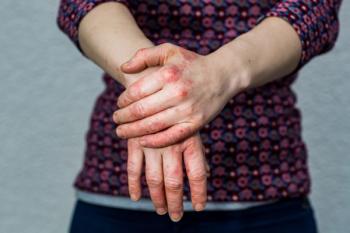
GlobalData has published a report that predicts the atopic dermatitis pipeline will see great transformation and growth in the next 10 years.

Mark Lebwohl, MD, explains the recent delay in approval of JAK inhibitors by the FDA, how it is affecting the inflammatory skin disease community, and if physicians should be concerned.
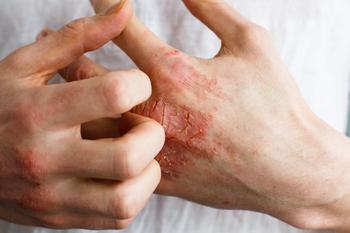
The results from the Heads Up study were published in JAMA Dermatology, which demonstrated upadacitinib was superior to dupilumab in treating atopic dermatitis.

A study from the Georgia Institute of Technology examined the difference of itch in hairy vs nonhairy skin.

Camp Wonder, a camp that supports kids with skin disease and is sponsored by Galderma, is celebrating their 20th year of operations this week.