
The European Commission has approved secukinumab (Cosentyx, Novartis) for treatment of moderate-to-severe plaque psoriasis in pediatric patients 6<18 years in the European Union.

The European Commission has approved secukinumab (Cosentyx, Novartis) for treatment of moderate-to-severe plaque psoriasis in pediatric patients 6<18 years in the European Union.

The U.S. Food and Drug Administration has approved atezolizumab plus cobimetinib and vemurafenib for treatment of BRAF V600 mutation-positive advanced melanoma. Studies show the combination treatment prolonged patients’ lives by 15 months without disease worsening.
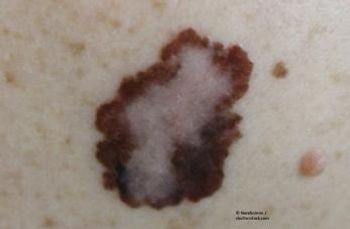
A recently published pooled 10-year analysis shows pembrolizumab demonstrates long-term survival benefits for advanced melanoma patients regardless of BRAF V600E/K mutation status or history of BRAFi, with or without MEKi therapy.

The U.S. Food and Drug Administration has approved Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%, MC2 Therapeutics) for adult with plaque psoriasis. Wynzora is equipped with the MC2 Therapeutics’ patented PAD Technology and is currently under review in the EU.

Guselkumab becomes the first IL-23 inhibitor approved for treatment of psoriatic arthritis by the U.S. Food and Drug Administration (FDA) following positive phase 3 results from two trials investigating the safety and efficacy of the drug.

The U.S. Food and Drug Administration has approved Mylan and Fujifilm Kyowa Kirin Biologics’ adalimumab-fkjp as a biosimilar to AbbVie’s adalimumab for a variety of inflammatory disease including plaque psoriasis and psoriatic arthritis.
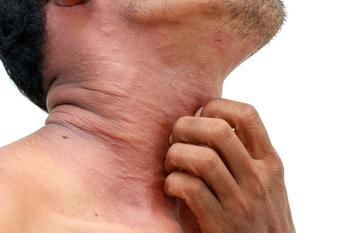
The U.S. Food and Drug administration has accepted the Biologics License Application for tralokinumab (LEO Pharma) for moderate-to-severe atopic dermatitis and is expected to make a decision in the second quarter of 2021.

The U.S. FDA has approved collagenase clostridium histolyticum-aaes (Qwo, Endo International), making it the first FDA-approved injectable treatment for moderate-to-severe cellulite in the buttocks in adult women.

The U.S. Food and Drug Administration has approved a single-dose 300 mg pre-filled pen version of dupilumab (Dupixent, Sanofi and Regeneron) for the treatment of atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis (CRSwNP) in U.S. patients 12 years and older.
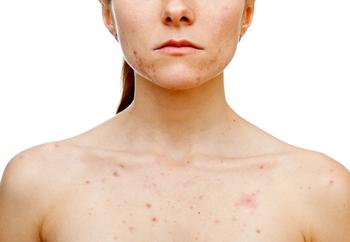
Almirall announces the U.S. Food and Drug Administration has approved the updated label for their oral antibiotic sarecycline (Seysara) for treatment of acne vulgaris to help promote the appropriate use of the drug to aid in preventing antimicrobial resistance commonly warned when using antibiotics

ICYMI, this week’s edition features a video series on social media use in dermatology, the launch of our new podcast, as well as articles about FDA approvals for acne and cSCC, progress in epidermolysis bullosa, new rosacea management treatment, plus more.
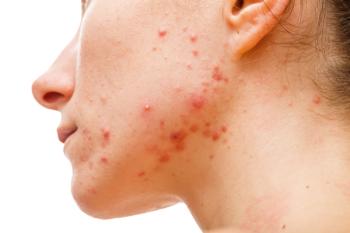
The U.S. Food and Drug Administration has approved the Abbreviated New Drug Application for topical retinoid adapalene gel USP, 0.3% from Alembic Pharmaceuticals and its joint venture Aleor Dermaceuticals for treatment of acne vulgaris.
Tazarotene lotion 0.045% (Arazlo, Ortho Dermatologics) is officially launched in the U.S. following approval by the U.S. Food and Drug Administration in late 2019 for the treatment of acne vulgaris in patients 9 years and older.

The U.S. Food and Drug Administration has approved pembrolizumab for cutaneous squamous cell carcinoma whose disease has shown to not be curable by radiation or surgery.

ICYMI, this week’s edition features articles about laser therapy for BCC, the growing interest in JAK-STAT inhibition, tips on how to sell your practice, apremilast for scalp pruritus, plus more.

ICYMI, we've curated a list of all the stories we've published throughout the past week. Check out this article for more Dermatology Times content.

LEO Pharma will soon begin marketing its monoclonal antibody tralokinumab for atopic dermatitis with the announcement that the EMA has accepted the marketing authorization application for the drug.
Dermavant announces the enrollment completion for their long-term safety study investigating tapinarof in adults with plaque psoriasis as a part of the PSOARING 1 and PSOARING 2 phase 3 program.