Atopic Dermatitis
Latest News
Latest Videos

CME Content
More News

Raj Chovatiya, MD, PhD, gives a sneak peek of what to expect in atopic dermatitis education at the 2024 American Academy of Dermatology (AAD) Meeting in San Diego, California March 8-12. Since the last meeting, the AAD has released new guidelines to treat the condition.

Researchers have found Staphylococcus aureus may trigger itchiness in atopic dermatitis, opening the door to new treatment approaches in the future.

Learn more about dupilumab-induced conjunctivitis rates and misconceptions, the thriving long-term efficacy of dupilumab over 3 years, and unraveling the itch-flare dynamic in abrocitinib treatment.

The FDA has also granted nemolizumab Priority Review for prurigo nodularis.

Tapinarof cream demonstrated highly statistically significant improvement in the primary end point of vIGA-AD treatment success over vehicle at week 8.

IGA, EASI, and Itch Endpoints Met in First In Human Phase 2 Trial of Zabalafin for Atopic Dermatitis
Interim data from the clinical trial was presented in a poster at the South Beach Symposium, with itch reduction demonstrating immediacy and long-term capability.

A discussion among roundtable participants revolved around the importance of effective communication, the impact of prolonged use of topical steroids, and the need for trust building in recommending novel treatment options.

Exposure led to an increase in total plasma IgE levels but reduced epidermal thickening, mast cell number, and plasma histamine levels in the early stages of AD.

Atopic dermatitis flares are about more than just skin symptoms and are difficult to quantify, according to a study that asked patients to describe them.
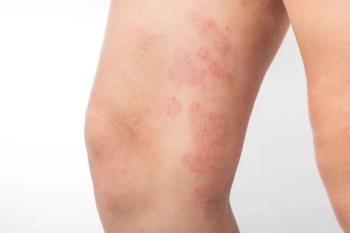
Researchers explored the efficacy of a multistep treatment strategy in a younger patient population.

Investigators highlighted the importance of considering household second hand smoke exposure when evaluating adolescents with AD.

In a review of relevant research, investigators found that in many aspects, telemedicine services were as effective as in-office care visits for AD.
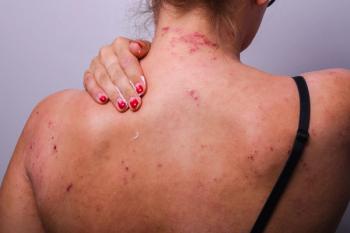
Functional magnetic resonance imaging response was utilized to test motor, somatosensory association cortex, perception, and sensory integration processing in a recent study.
Raj Chovatiya, MD, PhD, and Adelaide A. Hebert, MD, FAAD, review the study results for the ADORING 1 and ADORING 2 trials, as well as share closing thoughts on the use of tapinarof in management of AD.

Raj Chovatiya, MD, PhD, and Adelaide A. Hebert, MD, FAAD, comment on the tolerability and low discontinuation rates of tapinarof for patients in AD clinical trials, as well as discuss the adverse events associated with the drug.

In a recent Dermatology Times Case-Based Roundtable Meeting, Tina Bhutani, MD, MAS, shared intriguing cases and insights about atopic dermatitis treatment challenges faced by patients with different lifestyles and hobbies.
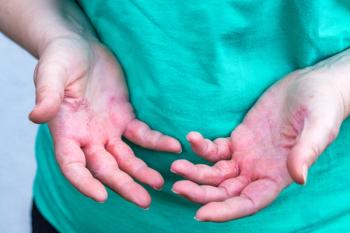
Caregivers and Patients With Atopic Dermatitis Prioritize Symptom Control, Adverse Effect Management
A study explored the prioritization habits of both patients with atopic dermatitis and their caregivers when it comes to treatment.
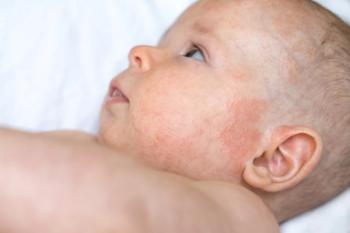
In a recent study, prenatal opioid exposure was also linked to increased odds of infections and asthma, but not with allergies, anaphylaxis, orautoimmune conditions.

Adelaide A. Hebert, MD, FAAD, shares insight on the eczema area and severity index 75 (EASI75) results from the ADORING 1 and ADORING 2 clinical trials for use of tapinarof in AD.

Expert dermatologists review the validated investigator global assessment for AD (vIGA-AD) response in the ADORING 1 and ADORING 2 trials for tapinarof.

An expert in dermatology reviews the case of a 33-year-old male patient suffering from atopic dermatitis for the past 3 years. He breaks down the panel's conversation on the use of non-steroidal topicals and emphasizes how patient assistance programs can ease burden for patients and providers.

Shawn Kwatra, MD, presents a case involving a 73-year-old African American female seeking treatment for her atopic dermatitis. In this segment Dr Kwatra explains the panels thoughts and rationale behind the prescribed treatments, discussing how each option works and its limitations.

The FDA has added efficacy and safety data for patients ages 12 years and older with uncontrolled moderate-to-severe atopic dermatitis with hand and/or foot involvement.

The pooled analyses from Arcutis’ INTEGUMENT-1 and INTEGUMENT-2 studies were presented at the 2024 Winter Clinical Hawaii Dermatology Conference.

Ten posters at the Winter Clinical Hawaii and Maui Derm Conferences will showcase investigational data to expand treatment options for patients with atopic dermatitis and chronic hand eczema.