Atopic Dermatitis
Latest News
Latest Videos
CME Content
More News
Functional magnetic resonance imaging response was utilized to test motor, somatosensory association cortex, perception, and sensory integration processing in a recent study.
Raj Chovatiya, MD, PhD, and Adelaide A. Hebert, MD, FAAD, review the study results for the ADORING 1 and ADORING 2 trials, as well as share closing thoughts on the use of tapinarof in management of AD.

Raj Chovatiya, MD, PhD, and Adelaide A. Hebert, MD, FAAD, comment on the tolerability and low discontinuation rates of tapinarof for patients in AD clinical trials, as well as discuss the adverse events associated with the drug.

In a recent Dermatology Times Case-Based Roundtable Meeting, Tina Bhutani, MD, MAS, shared intriguing cases and insights about atopic dermatitis treatment challenges faced by patients with different lifestyles and hobbies.
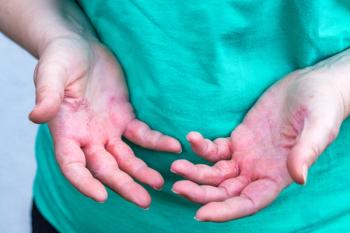
Caregivers and Patients With Atopic Dermatitis Prioritize Symptom Control, Adverse Effect Management
A study explored the prioritization habits of both patients with atopic dermatitis and their caregivers when it comes to treatment.
In a recent study, prenatal opioid exposure was also linked to increased odds of infections and asthma, but not with allergies, anaphylaxis, orautoimmune conditions.

Adelaide A. Hebert, MD, FAAD, shares insight on the eczema area and severity index 75 (EASI75) results from the ADORING 1 and ADORING 2 clinical trials for use of tapinarof in AD.

Expert dermatologists review the validated investigator global assessment for AD (vIGA-AD) response in the ADORING 1 and ADORING 2 trials for tapinarof.

An expert in dermatology reviews the case of a 33-year-old male patient suffering from atopic dermatitis for the past 3 years. He breaks down the panel's conversation on the use of non-steroidal topicals and emphasizes how patient assistance programs can ease burden for patients and providers.

Shawn Kwatra, MD, presents a case involving a 73-year-old African American female seeking treatment for her atopic dermatitis. In this segment Dr Kwatra explains the panels thoughts and rationale behind the prescribed treatments, discussing how each option works and its limitations.

The FDA has added efficacy and safety data for patients ages 12 years and older with uncontrolled moderate-to-severe atopic dermatitis with hand and/or foot involvement.

The pooled analyses from Arcutis’ INTEGUMENT-1 and INTEGUMENT-2 studies were presented at the 2024 Winter Clinical Hawaii Dermatology Conference.

Ten posters at the Winter Clinical Hawaii and Maui Derm Conferences will showcase investigational data to expand treatment options for patients with atopic dermatitis and chronic hand eczema.

Raj Chovatiya, MD, PhD, and Adelaide A. Hebert, MD, FAAD, discuss the rapid itch reduction results found in the clinical trials for tapinarof in AD, as well as primary end points of the studies.

Expert dermatologists highlight the patient characteristics in the ADORING 1 and ADORING 2 trials, both focusing on tapinarof for the treatment of AD.

Innovative practice pearls for both vitiligo and atopic dermatitis treatment were shared with nearly 200 clinicians to put into practice at the virtual Revolutionizing Vitiligo and Revolutionizing Atopic Dermatitis Conferences.

The additional efficacy data highlights tapinarof cream 1% and its impact on EASI, PP-NRS, ViGA-AD, and more.

Study findings suggest that Lavandula species, with their historical use in folk medicine, could emerge as valuable additions to AD treatment regimens. However, it's crucial to note that the efficacy of these oils is concentration-dependent.

Test your knowledge and explore a recent study on the relationship between alcohol consumption and skin diseases, including atopic dermatitis.
The topical therapy was more effective in patients with psoriasis and AD when compared to a vehicle control cream.

Raj Chovatiya, MD, PhD, and Adelaide A. Hebert, MD, FAAD, review clinical trial data for tapinarof cream, 1% in the treatment of AD.

Expert dermatologists discuss the use of nonsteroidal treatment formulations for the treatment of AD, focusing on a topical treatment, tapinarof cream, 1%.

A late breaking session from Revolutionizing Atopic Dermatitis Virtual Conference analyzed the frequency and clinical meaningfulness of conjunctivitis.

As 2023 comes to a close, Dermatology Times is taking a look back at the studies, treatment, and advances in atopic dermatitis this year.

An expert in the management of skin conditions highlights the case of a 45-year-old female with severe pruritus, erythematous rash, and lichenification across multiple body areas, worsened by recent work-related stress from a promotion. Dr. Lio offers insights into managing such patients and shares practical tips for a streamlined prescription process.