
Patients with CHE saw higher treatment success and a favorable safety profile with delgocitinib 20-30 mg/g.
Marie Bosslett is the assistant editor of Dermatology Times® and joined the MJH Life Sciences team in October 2024. She attended Loyola University Maryland in Baltimore and earned a Bachelor of Arts in Communication with minors in marketing and writing. When she’s not writing, Marie enjoys going to concerts, reading on the beach, and spending time with friends and family.

Patients with CHE saw higher treatment success and a favorable safety profile with delgocitinib 20-30 mg/g.

Two-sample Mendelian randomization analysis explored lifestyle factors such as sleep, smoking, alcohol consumption, and sedentary behavior.

The therapy, which combines clindamycin phosphate, adapalene, and benzoyl peroxide, was effective for Hispanic patients with moderate to severe lesions.

In a prospective study, patients with scalp seborrheic dermatitis saw positive results in efficacy, safety, and quality of life when using the AgNP product.

Treatment with tildrakizumab was still safe and effective after 1 year with notable improvements in patients’ quality of life.
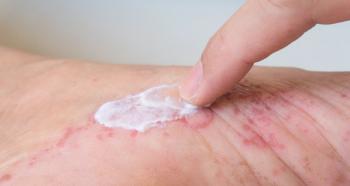
Use of the product showed positive changes in the skin’s hydration, lipid content, transepidermal water loss, and erythema.

When compared to a non-active product, the formula improved texture, reduced erythema, and strengthened the skin barrier.

The novel treatments will be for patients with orphan conditions like Netherton Syndrome, Peeling Skin Syndrome, SAM Syndrome, and palmoplantar keratoderma.

Each treatment had benefits and disadvantages for patients who struggle with rosacea-associated erythema.

Participants from the parent DELTA 1 and 2 studies maintained their disease control and observed a decrease in pain and itch over 52 weeks of treatment.

As a featured clinician in EltaMD’s Derm Day campaign, Mona Gohara, MD, spoke about her connection with the brand and the work being done to unify dermatologists and promote skin health.

In case you missed it, this week we had news about positive results from Incyte’s phase 3 STOP-HS trials, multidisciplinary approaches to advanced BCC and CSCC, pediatric ocular adverse effects associated with dupilumab, and more.

Eve Arnera and Thandiwe Munaiwa, MD, spoke about the groundbreaking collaboration between SkinCeuticals and ReSurge International, “Pioneering Women in Reconstructive Surgery.”

The 730-nm picosecond laser had an efficacy rate of 88% with higher patient satisfaction compared to the 1604-nm wavelength.

This real-world data explores the use of the IL-17A blocker for up to 3 years in several subtypes of psoriasis.

John Strasswimmer, MD, PhD, spoke on the difficulty of managing adverse effects, the role of interdisciplinary clinical relationships, and the challenges in complex and older patients.

Participants experienced smoother, younger-looking skin after just 1 session, with results lasting up to 16 weeks.

According to a survey, both patients and clinicians are unsatisfied with the current therapeutic landscape and are looking for options that better fulfill patient needs.

According to a clinical trial and in vitro study, TTFE can promote scalp health and hair growth by 96.88% while reducing inflammation.

A comprehensive evaluation of therapies found that lasers were most effective with high rates of patient satisfaction.

Patients observed a 56% reduction in lesions after 56 days of treatment with only mild adverse events.

In case you missed it, this week we had news about the FDA voluntary benzoyl peroxide recalls, the National Eczema Association's strategic efforts, recaps of this week’s AAD Annual Meeting, and more.
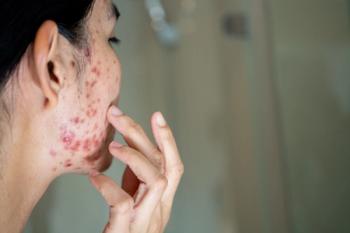
Red meat, chocolate, milk, and foods with a high glycemic index may impact the presence of acne in patients.

99% of posts on the platform were of poor quality, according to the DISCERN instrument.

A majority of patients noticed improvement in hyperpigmentation spots, skin texture and evenness after 12 weeks of use.

Nearly all patients in a new study saw an improved appearance in their forehead up to 12 months after treatment.

Compared to placebo, there was a 70% reduction in IgE levels in children ages 0.5 to 6 after 16 weeks of therapy.
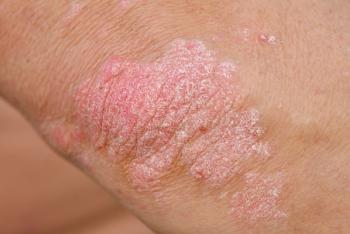
New results from Alumis show a positive clinical response and safety profile after 52 weeks of treatment for patients with plaque psoriasis.

Efficacy, safety, and pharmacokinetic data support the use of vilobelimab in treating pyoderma gangrenosum and hidradenitis suppurativa.
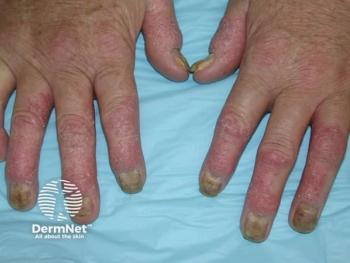
According to a poster from AAD, the National Psoriasis Foundation’s treatment goals were used as a benchmark to determine the risk of developing psoriatic arthritis after initiating biologics.