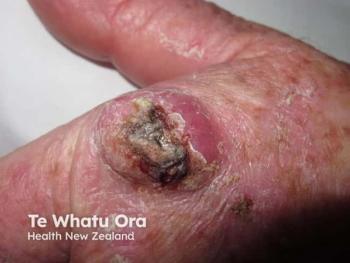
Skin rash post target therapy may be a “cytokine storm”
Doctors suggest that cytokine antagonists may lessen the severity of the rash.
Around one in five melanoma patients treated with BRAF/MEK inhibitors after previous immunotherapy present with a severe skin rash (grade ≥ 3), which may be complicated by systemic involvement and organ damage.ii
It has been previously suggested that those with kidney, liver, and heart involvement experience a systemic reactionâdrug rash with eosinophilia and systemic symptoms (DRESSiii)â but now researchers writing in a letter to the journal Melanoma Research have proposed that patients experience a “cytokine storm” and that their symptoms are actually due to cytokine release syndrome (CRS).iv.
The research group has previously reported four cases of cytokine release syndrome (CRS) following the use of BRAF/MEK inhibitors after immune checkpoint blockade with severe (grade ≥3) skin and systemic involvement.v vi The pattern of the symptomsâpyrexia and maculopapular rash, and also transaminitis (an uncommon event during treatment with BRAF/MEK inhibitors) âfulfils the diagnosis of a cytokine release syndrome, says one of the researchers Reinhard Dummer, M.D., University Hospital of Zurich, Switzerland. “The documented, high circulating cytokine levels, including interleukin-6 (IL-6), tumor necrosis factor-α, and interferon-γ, further confirm our hypothesis,” he said.
Such episodes have also been reported in the preliminary findings of a phase three clinical trial with dabrafenib, trametinib, and an anti-PD1 antibody.vii
Dr. Dummer points out that these cases are distinguished from a hypersensitivity syndrome to the kinase inhibitors (DRESS) as they lack of frequently observed eosinophilia, other blood abnormalities, reactivation of human herpes virus, or lymphadenopathy do not support the diagnosis. The short onset of symptoms after treatment initiation (around 10 days) and the tolerance of the identical medication after reinduction are also typical for a systemic inflammatory reaction and untypical for DRESS, he adds.
“We attribute this systemic reaction to an aberrant release of proinflammatory cytokines, mainly accompanied by symptoms such as pyrexia, transaminitis, renal insufficiency, and maculopapular skin rash,” he says.
The high level of immune activation in the context of simultaneous inhibition of checkpoint and MAPK pathway inhibition means a ‘cytokine storm’ is a possible mechanism for CRS. Dr. Dummer suggests. “Oncogenic drivers in the immune system leads to a T-cell activation and consecutive release of cytokines, such as tumor necrosis factor-α and interferon-γ, with further activation of innate immune cells and production of numerous cytokines, among all also IL-6, which importantly causes a positive feedback loop, leading to a ‘cytokine storm. However, clinical and preclinical data propose that MAPK pathway inhibition may enhance T-cell activation through an increase of proinflammatory cytokines and cytotoxic factors. These changes to the tumor microenvironment would be expected to increase efficacy by making tumors more susceptible to immunotherapy.”
The management and treatment of the CRS can be very challenging. “We advise the practitioners to measure the cytokine levels and use cytokine antagonists as potential therapeutic candidates in cases refractory to steroids,” Dr. Dummer says.
REFERENCES
i. Lamiaux M, Scalbert C, Lepesant P, Desmedt E, Templier C, Dziwniel V, et al. Severe skin toxicity with organ damage under the combination of targeted
therapy following immunotherapy in metastatic melanoma. Melanoma Res 2018; 28:451–457.
ii. Lamiaux M, Scalbert C, Lepesant P, Desmedt E, Templier C, Dziwniel V, et al. Severe skin toxicity with organ damage under the combination of targeted
therapy following immunotherapy in metastatic melanoma. Melanoma Res 2018; 28:451–457.
iii. Lamiaux M, Scalbert C, Lepesant P, Desmedt E, Templier C, Dziwniel V, et al. Severe skin toxicity with organ damage under the combination of targeted
therapy following immunotherapy in metastatic melanoma. Melanoma Res 2018; 28:451–457.
iv. Dimitriou F, Mangana J, Micaletto S, Braun RP, Dummer R. Cytokine release syndrome as an important differential diagnosis of severe skin toxicity with organ damage during switch from immunotherapy to targeted therapy in metastatic melanoma. Melanoma Res. 2019 Feb;29(1):107-108. doi: 10.1097/CMR.0000000000000544.
v. Dimitriou F, Matter AV, Mangana J, Urosevic-Maiwald M, Micaletto S, Braun RP, et al. Cytokine release syndrome during sequential treatment with
immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J Immunother 2018. [Epub ahead of print].
vi. Urosevic-Maiwald M, Mangana J, Dummer R. Systemic inflammatory reaction syndrome during combined kinase inhibitor therapy following anti-PD-1
therapy for melanoma. Ann Oncol 2017; 28:1673–1675.
vii. Dummer R, Arance Fernández AM, Hansson J, Larkin JMG, Long GV, Gasal E, et al. Preliminary findings from part 1 of COMBI-i: a phase III study of anti-PD-
1 antibody PDR001 combined with dabrafenib (D) and trametinib (T) in previously untreated patients (pts) with advanced BRAF V600-mutant melanoma. J Clin Oncol 2018; 36 (Suppl):189.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.