|Articles|January 1, 2004
FDA panel recommends label change
Author(s)John Jesitus
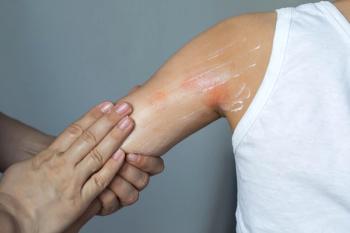
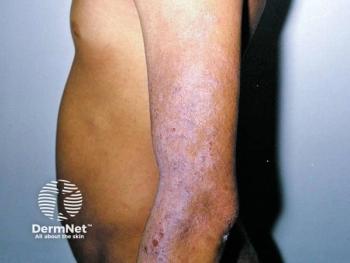
Washington, D.C. - Responding to concerns over increased malignancies seen in animal studies, a pediatric subcommittee of the FDA's Anti-Infective Drugs Advisory Committee said topical immunosuppressant labeling should include a warning against use in children under the age of two.
Advertisement
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.
Advertisement
Latest CME
Advertisement
Advertisement