FDA approves topical for actinic keratosis
Tirbanibulin (Klisyri, Almirall) has been approved by the U.S. Food and Drug Administration (FDA) for the topical treatment of actinic keratosis on the face or scalp.
The U.S. Food and Drug Administration (FDA) has approved tirbanibulin (Klisyri, Almirall) for the topical treatment of actinic keratosis (AK) on the face or scalp, according to a company press release.1
Tirbanibulin, a novel, topical first-in-class microtubule inhibitor, will be launched in the US during the first quarter of 2021, according to Almirall.
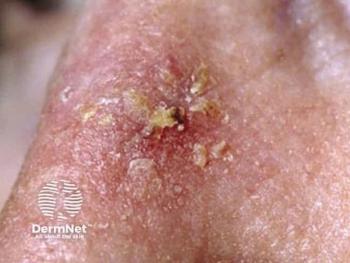
AK is the second most common diagnosis made by dermatologists in the United States; AK has a reported a prevalence of between 11% and 25%. Early diagnosis and treatment is imperative, as AKs can progress into squamous cell carcinoma.
The approval is based on data from one of the largest phase 3 clinical study programs conducted for a topical AK treatment, as well as 2 double-blind, vehicle-controlled phase 3 studies (KX01-AK-003 and KX01-AK-004). These studies enrolled a total of 702 patients across 62 sites in the US. According to the results, tirbanibulin achieved a significantly higher number of patients with complete clearance of AK lesions in the treated area compared with vehicle (44% vs 5% in study 1 and 54% vs 13% in study 2). Tirbanibulin also met the secondary end point of partial clearance of lesions.
“In addition to proven efficacy, Klisyri demonstrated safety, with the most common adverse events (AEs) being application site pruritus and pain seen in 9% and 10%, respectively of patients treated with it,” Ayman Grada, MD, head of R&D and Medical Affairs for Almirall US said in a statement. “Of note, no patients withdrew from the study due to AEs.
Tirbanibulin is supplied in boxes of 5 single-use sachets and is applied to the treatment area once daily for 5 days, which is the shortest application period of any topical treatment for AK, according to Almirall.
This article was initially published by our sister publication,
Reference:
- Almirall announces FDA approval of Klisyri (tirbanibulin), a new innovative topical treatment for actinic keratosis. News release. Almirall; December 15, 2020. Accessed December 15, 2020.
https://www.almirall.com/newsroom/news/almirall-announces-fda-approval-of-klisyri%C2%AE-tirbanibulin-a-new-innovative-topical-treatment-for-actinic-keratosis
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.